Sharks and llamas share a strange quirk of their immune systems. Hayley Bennett finds out how their ‘nanobodies’ could help us tackle Covid and a host of other diseases
Around town, US pharmacologist Aaron LeBeau is ‘the shark guy’, a strange nickname for someone who develops cancer therapies for a living. When LeBeau accepted a job with the University of Wisconsin–Madison last year, the deal included an 8000-gallon (30m3) saltwater shark tank, which is now complete with four beautiful baby nurse sharks. ‘It’s the world’s worst kept secret,’ he says. ‘I met my neighbour for the first time and she’s like “Are you the guy with the sharks?”’
Until recently, LeBeau was in Minnesota draining blood from camels. The connection? Despite apparently sharing nothing in common, sharks and camels are united by a curious quirk of evolution that humans are exploiting to tackle everything from Covid to cancer.
The story begins in the 1980s, with a group of Belgian researchers who were developing a test for sleeping sickness in water buffaloes. All the test required was a few drops of buffalo blood. Then, as Serge Muyldermans from the Vrije Universiteit Brussel in Belgium explains, they heard from a former student working in Mali who wanted to try the same test on camels. The student sent over some Arabian camel blood and the team used a couple of drops to prove that the test also worked in dromedaries, then put the leftover blood in the refrigerator.
The students kept coming up with a fraction that wouldn’t separate – either they were doing something wrong or these camels had some very odd antibodies
Soon after, Muyldermans was setting up practicals for some biology students that involved taking blood from a human volunteer and isolating out the antibodies in it via some standard lab techniques – affinity chromatography to purify them and gel electrophoresis to separate them by molecular weight. Antibodies are traditionally thought of as being made up of two types of protein chains: ‘heavy’ and ‘light’ chains, the former being unsurprisingly bulkier and heavier. Two heavy chains pair with two light chains to form one immunoglobulin molecule – the classical Y-shaped antibody that we produce to defend ourselves from viruses, bacteria and other invaders, either as a result of vaccination or after an infection. It’s an unmistakable structure to anyone who has ever studied immunology. The two heavy chains make up the double-thickness trunk of the Y, peeling apart at the top like outstretched arms to which the light chains are stuck like arm plates to a suit of armour.
The idea was that the students would use chemical reducing agents to break up their Y-shaped antibodies and then separate them by molecular weight into heavy and light chains. But as Muyldermans recalls, the students refused to do it at first. ‘Their argument was that it was dangerous because they were not sure about the blood donors – they might be infected with HIV or hepatitis,’ he says. So, after some arguments about mouse blood, Muyldermans offered them the camel blood still sitting in the fridge. Finally, the practical went ahead, but not as expected. The students kept coming up with a fraction that wouldn’t separate. It appeared to be heavy chain only – no arm plates. This must mean one of two things: either they were doing something wrong or these camels had some very odd antibodies.
It was his colleague, the late Raymond Hamers, who argued there could be something more to it than just experimental error. But Muyldermans was immediately excited about what it could mean for his own work, which involved making mouse antibodies in bacteria. By then, when he managed to get the bacteria to make them, he couldn’t get the heavy and light chain proteins properly folded and linked together. ‘So when we realised that perhaps the camels’ antibodies had no light chain, I was especially interested because half of my problems were solved,’ he says.
Immunity in miniature
It took some work to prove that the atypical antibodies were functioning parts of the camels’ immune systems but by 1993, the team was reporting their surprising discovery in Nature. As it turns out, camelids like camels and llamas have ‘normal’ antibodies like ours as well as these light-chain-less antibodies or ‘nanobodies’, which lack their arm plates but also have shorter arms, making them much more compact – around 75kDa compared to 150kDa.
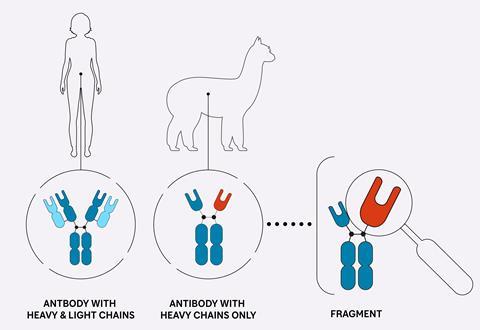
What was intriguing about this discovery was that the camelids appeared to have tinkered with a system that had worked well enough for millions of years. In other animals, it’s the paired heavy–light chain structure that allows us to bind billions of different targets on invading microbes. This structure is the result of the mixtape -like reassortment of the gene segments that produce both the heavy and light protein chains during immune cell development. The classical antibody’s immune repertoire is akin to a playlist compiled from two music collections, each containing thousands of tracks, while a nanobody’s is created from just one collection. But, as Muyldermans explains, it somehow works: ‘It’s less diverse compared to the heavy–light combinations, but still it can compete to generate a good immune response.’
Camel antibodies are better at neutralising viruses
Evolutionarily speaking, though, it’s still puzzling: how, and why, did these pared-down antibodies arise? Cory Brooks, a structural biologist at California State University in Fresno, US, addressed the how in a 2018 paper, suggesting it was an evolutionary accident due to changes in sections of the heavy chain where it interacts with the light chain – basically, the bit that stuck the two together came undone. But if camels use ordinary antibodies as well, then what’s the benefit? According to Brooks, they seem to be ‘better at neutralising viruses’ than regular antibodies. ‘Camels do have a large viral load in them and the viruses don’t seem to do much,’ he says. ‘If those same viruses infect other ruminants, the animals get sick, but the camels are OK.’ Both Brooks and Muyldermans wonder whether there was some deadly virus in camelid history that these smaller antibodies helped to fight off.
What’s fascinating, though, is that this system hasn’t just cropped up once in evolutionary history, but at least twice, as Andrew Greenberg at the University of Miami, US, and colleagues revealed in 1995. Sharks use single-chain antibodies called immunoglobulin new antigen receptors (IgNARs) that are even smaller – and more stable, according to LeBeau – than camelid antibodies. This system, common to sharks, rays and other cartilaginous fish, arose completely independently, suggesting it provides some advantage. Perhaps in both cases, Brooks’ work suggests, the sturdiness of the smaller molecules offers some protection against harsh environments – high body temperatures in the case of camelids or high urea concentrations in the case of sharks and their relations, as they retain urea in their blood to balance the high salt concentrations in seawater.
Five years ago, it was camelid antibodies LeBeau was working on at the University of Minnesota, where he was using them to hit cancer cell targets, for imaging and to develop treatments. But after a couple of years of doing this and seeing that it worked beautifully, a graduate student suggested they give the shark versions a go. Back then, LeBeau wasn’t sure how he was going to get his hands on any shark blood. ‘We are in the middle of the United States,’ LeBeau told him. ‘There are zero sharks around here.’ They made enquiries at a local aquarium, who were happy to oblige, but at 300kg per fish, LeBeau didn’t fancy his chances taking a sample. At this point, LeBeau could have given up and built a llama farm.
Pharma llama farmers
But that’s just what French pharmaceutical giant Sanofi did. And some of their treatments, the first based on heavy-chain only antibodies, are starting to hit the market. Sanofi bought the start-up Ablynx, a spin-out from Muyldermans’ institution and the Vlaams Instituut voor Biotechnologie in Belgium, for €3.9 billion (£3.4 billion) in 2018. Since then it has been developing nanobodies that it links together to make multi-pronged therapeutics. Many of these come initially from immunising llamas with the target molecule for whatever disease the company is interested in, waiting for 6–12 weeks, then drawing blood and using the same target molecule to select and enrich for the best binders.
This process, known as ‘panning’, gets its name from the ancient small scale gold mining technique. ‘It’s the same thing as getting a big pewter plate and going into the soil trying to find the densest piece in a bunch of silt,’ LeBeau says. It’s not unique to Sanofi; similar strategies have been used elsewhere to find camel antibodies that bind breast cancer targets, for example, and components of scorpion venom. Synthetic libraries of camel and shark antibodies can also be panned but it’s thought that using live animals cuts down on the work of sifting out the best binders, since their immune systems do some additional molecular legwork to optimise their binding structures after they’ve met their target. But each approach has its challenges, points out Pieter Deschaght, project head for global innovation at Sanofi – looking after a farm full of llamas isn’t exactly an easy option.
Nanobodies can reach the parts of their target molecules that larger traditional antibodies can’t
So what has the company got in its pipeline? Its first-in-class drug caplacizumab, a nanobody targeting blood proteins involved in a rare blood disorder, was approved in the EU in 2018, with US approval following the year after, and it appears there is plenty more to come. Sanofi’s current targets include errant molecules in our own immune systems in diseases like rheumatoid arthritis and lupus. Another Ablynx/Sanofi drug, ozoralizumab, has been licensed to Japanese company Taisho Pharmaceutical, which announced positive results in clinical trials for rheumatoid arthritis in December. This ‘trivalent’ drug is example of the multi-pronged approach, linking together two nanobodies binding to tumour necrosis factor alpha (TNF-alpha) and one binding to a common blood protein, which helps to extend its stay in the body. TNF-alpha antibodies are already a prominent feature of autoimmune treatments, but the contention is that this smaller nanobody version should penetrate deeper into tissues and trigger fewer immune reactions to the drug itself.
It’s the small size, robustness and ‘formatting flexibility’ of these nanobodies that makes them so unique and valuable, according to Deschaght. They can also be cloned and produced at scale in bacteria, as Muyldermans hoped, or in yeast and mammalian cells. But one of the chief reasons that nanobodies are so advantageous, Deschaght says, is that they can reach the parts of their target molecules that larger traditional antibodies can’t. ‘That’s a big difference,’ he says. ‘So you can go for other targets with nanobodies compared to conventional antibodies.’ This is a direct consequence of their size – they can fit into smaller gaps – but it may also be due to a unique aspect of their structure. Antibodies have finger-like projections at the ends of their arms, where they make contact with their targets (see box Hot bond below), but in camelids one of these is longer than in other animals. It’s commonly claimed that this allows them to reach further into crevices on a given target molecule’s surface, accessing ‘cryptic epitopes’ or hidden binding regions.
Brooks, who uses crystallography to study the molecular structures involved in these interactions, says there’s ‘pretty strong’ evidence for this, at least when the targets are viral, as in HIV and Covid, for example. ‘It’s true that they do access these hidden epitopes,’ he says. But he points out that without directly comparing how well regular antibodies and nanobodies bind the same targets, it’s a claim that’s difficult to prove. To Brooks’ mind, infectious diseases including viruses are probably some of the best targets to chase where nanobodies are concerned, particularly considering the hypothesis that they evolved as ‘virus neutralisers’ in camelids. Whether Sanofi is on the trail of viral targets, however, Deschaght can’t say. However, he does agree there is ‘a lot of potential for nanobodies for infectious diseases such as Covid’ as well as ‘across all disease areas’.
Hot bond
Y-shaped antibodies make contact with their molecular targets using probing, finger-like structures that protrude from the tops of their outstretched arms. In conventional antibodies, these fingers, known as complementarity determining regions (CDRs), are formed from loops in two protein chains – both the heavy and light chains. In camelids’ heavy-chain-only antibodies, however, they are formed solely from the heavy chain, and contain three loops instead of six. In camelids, the third finger is longer than in humans. One of the more subtle differences, though, is an extra disulfide bond in camelids, between the first and the third finger. This additional bond has become a subject of some debate. ‘Honestly, it’s a mystery why it’s there,’ says Brooks.
It has been suggested that the bonus bond might stabilise the longer third finger as it contacts the antibody’s target. ‘There’s this hypothesis that because the binding loop is a lot longer, if you put this disulfide in, that restricts the loop and that helps with binding,’ Brooks says, adding that in other antibodies with giant CDR loops (as in cows) this is almost definitely the case. But when he and his colleagues at California State University, Fresno, in the US tested this theory in 2020, by deleting the extra disulfide in camel antibodies for Listeria, they didn’t see much difference in how well the antibodies bound to the bacteria. At the very least, this suggested the effect wasn’t universal.
So is there another explanation? What the study did show was that when the researchers removed the bond and then heated up the antibodies, they unfolded and clumped together – even after cooling they couldn’t reform. Yet with the bond intact, most were still able to refold. This, along with the realisation that the bond was almost exclusively found in camels and not llamas or alpacas, led the researchers to the curious conclusion that the bonus bond could be a ‘niche adaption’ to hot climates. Camels maintain a higher body temperature (40°C) than animals in cooler climates in order to prevent water loss by evaporation. By stabilising their antibodies with additional bonds, they may just be saving them from damage in the desert heat.
At the shark end

For Sanofi, building a llama farm was preferable to keeping sharks, but for LeBeau – who claims he finds immunology ‘boring’ – sharks is where it gets interesting. Not long before the pandemic hit, he started talking to Caroline Barelle, chief executive of Elasmogen, a Scottish company developing single-chain ‘soloMER’ antibodies as drugs. Elasmogen had been bleeding sharks to build a synthetic library containing billions of shark antibodies and was looking for academic collaborators to work with on cancer therapies. Immediately after their first chat, LeBeau began looking for shark tanks and in February 2020 met Barelle in London, where they discussed joint projects. ‘And then Covid-19 came about,’ LeBeau says. ‘So she went back to Scotland, I went back to Minnesota, and she called me up and said “I want to make Covid-19-neutralising [antibodies ].”’
You can mutate 95% of the Covid-19 spike protein and one of our shark antibodies was still working
Working together, they went through Elasmogen’s synthetic library and found a bunch of binders for the Covid-19 spike protein, then probed the finer details of their binding interactions by carrying out crystallographic analyses back in Minnesota. As LeBeau recalls, the structural work showed the ‘crazy molecular yoga’ that the tiny shark antibodies were doing to get into areas of the spike protein that even llama antibodies couldn’t reach. In tests against the live virus, the team claims they were at least as good as human anti-Covid-19 antibodies at neutralising the virus and better than camelid antibodies.
This was last year. With the virus evolving so quickly, however, what’s more important is their apparent resistance to new variants, as LeBeau explains. ‘We’ve done subsequent studies and we found that these shark antibodies were highly effective against omicron,’ he says, referring to the more infectious variant, containing at least 30 changes in the spike protein, which emerged in South Africa in November 2021. ‘We even did some molecular modelling and found that you can mutate like 95% of the spike protein and one of our [shark] antibodies was still working.’
Their antibodies appear to have a key advantage over our own cumbersome Covid-19 binders. Human antibodies directly recognise the region of the spike protein that contacts human cells, a region that is always under pressure to adapt and mutate. Shark antibodies, on the other hand, sneak underneath it into a region that according to LeBeau ‘never mutates’ – and if it does then it’s highly conserved, even as far back as the Sars variant that hit Asia in 2003. Interestingly, they also work against coronaviruses from wild animals, which also have this conserved region, and could one day spill over into humans. So could they be useful for a future outbreak? It’s possible. They’re currently being tested in mice, with the hope that when the next big outbreak comes, they’ll be ready to roll.
While all of this was going on, LeBeau was still eyeing up shark tanks. Finally, in 2021, he got the offer from Wisconsin, where he’s now immunising live sharks with cancer targets. Colleagues are working on coronavirus, while Elasmogen is pursuing targets for arthritis and inflammatory bowel disorders. The possibilities seem endless – from haemorrhagic fever to vaccines against drug abuse.
For someone who is ostensibly a cancer researcher, LeBeau seems pretty enamoured with sharks and their strange antibodies. ‘They’re just amazing, weird little proteins,’ he says. The second, bigger tank is already on order.
Hayley Bennett is a science writer based in Bristol, UK













No comments yet