‘Easy-to-make’ reagent adds a wide variety of functional groups to aromatic rings
Chemists planning to synthesise organic molecules may want to go back to their drawing boards, because a team in Spain has developed a new way to form carbon–carbon bonds. Marcos Suero’s team from the Institute of Chemical Research of Catalonia (ICIQ) in Tarragona, Spain has exploited diazomethyl reagents equivalent to carbynes, carbon atoms with three non-bonded electrons.
The ICIQ team forms three bonds, where the orientation of groups around a carbon atom differentiates isomers that otherwise have the same composition. The approach adds ‘a new Lego piece in retrosynthetic analysis’, Suero tells Chemistry World.
Over 40 years ago, researchers showed that a carbyne’s non-bonded electrons divide into a pair – a carbene – and a lone radical. But until now, no-one knew how to make these highly reactive substances controllable. ‘We have revived a forgotten reactive species,’ Suero says.
The key to the ICIQ team’s process is a hypervalent iodine reagent that provides the carbyne-equivalent diazomethyl radical. This lets the ICIQ team use the radical and carbene aspects of the carbyne’s nature selectively and separately. They use the single radical nature of this intermediate to form a carbon–carbon bond to aromatic rings, and exploit the carbene aspect for further transformations.
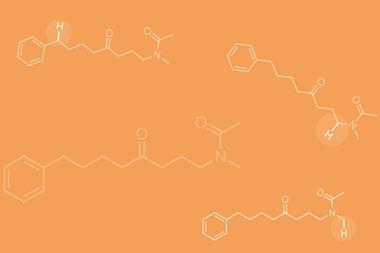
Together with a ruthenium catalyst, in a cheap reactor surrounded by white LED strips, they exploit this to add diazomethyl groups to many aromatic ring structures. They also swapped the white LEDs for blue ones, and added a variety of extra reagents to the hypervalent iodine reagent and ruthenium catalyst. That added three bonds, generating chiral products including amino acids, alkyl halides and cyclopropanes. Suero says the process is simple, and the hypervalent iodine reagent is easy to prepare. Although there may be concerns about diazomethyl groups being explosive, the team’s research indicates that they are safe at room temperature.
The process doesn’t yet work with basic nitrogen groups like amines, and Suero admits this is ‘an important problem to solve’ because they’re common in drug molecules. His team is now also exploring carbynes’ unique fundamental reactivity.
‘This is a truly lovely piece of work, nicely conceived and executed,’ says the University of Cambridge’s Steve Ley. ‘It leads to new carbon–carbon bonds and provides a set of very useful reactive building blocks. The substrate scope is very impressive and I can see this work being applied rapidly by groups across the world, particularly for late stage functionalisation programmes.’
References
Z Wang et al, Nature, 2018, DOI: 10.1038/nature25185












No comments yet