N-heterocyclic carbene-borane complexes with a rare stereogenic boron centre demonstrate potential in stereoselective catalysis
Researchers have developed a library of chiral N-heterocyclic carbene (NHC)-boranes that are stereogenic at the boron atom. These complexes are formed in an excellent diastereomeric ratio without the need for chiral separation, and may lead to new stereoselective catalysts.
Difficulty in handling and controlling its reactivity means that organic chemists typically overlook boron. However, it has recently undergone a resurgence in popularity due to its unusual activity as both an electrophile and a Lewis acid.
While p-block elements such as boron can serve as effective catalysts, for example in the form of a frustrated Lewis pair (FLP), there have been few reports of chiral boranes. Those previously reported are typically prepared as racemic mixtures, which are then separated by chiral HPLC – a time consuming and expensive process. The direct diastereoselective synthesis of chiral boranes is rare.
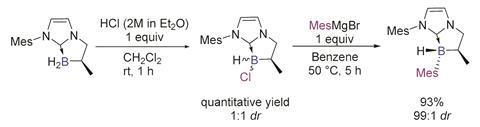
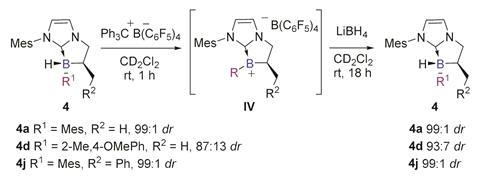
Olivier Chuzel and his team in Aix-Marseille University, France, have developed a new way to synthesise stereogenic boron centres directly in the form of NHC-boranes. By having a neighbouring stereogenic carbon atom in the molecule during the C–B bond formation step, Chuzel’s team obtained a library of these boranes in a diastereomeric ratio of up to 99:1.
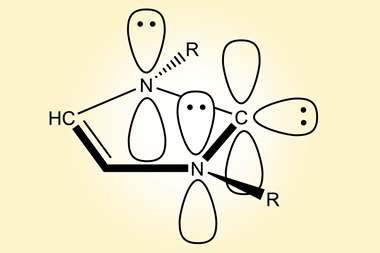
Chuzel explains that the difficulty in controlling the tetra-coordinated boron centre comes from the labile nature of the attached ligands. ‘The racemisation barrier decreases dramatically in the case of weak ligands, and the boron atom becomes configurationally unstable,’ he explains. ‘NHCs are good σ-donors that increase the strength of the C–B bond, making them quite resistant to dissociation.’
Not only are these NHC-boranes stable, but the chiral boron centre is also regenerated stereoselectively by hydride addition to a planar borenium intermediate. They have only been briefly demonstrated as catalysts showing slight enantioselectivity in the hydrosilation of an imine, but the stability and regeneration of these complexes opens up significant opportunities for further applications.
‘I expect that we will see more applications of these boranes in the future, especially in the field of metal-free enantioselective catalysis,’ says Rebecca Melen of Cardiff University, UK, an expert in main group catalyst design. ‘One of the most demanding tasks for the synthetic chemist in designing speciality chemicals, particularly in the development of pharmaceuticals, is to control the orientation of atoms in molecules during bond forming reactions.’
Chuzel hopes the work will open up the possibilities for stereoselective FLP-type reduction reactions. His group is currently focusing on the enantioselective reduction of unsaturated substrates by this method.








No comments yet