Scientists in China and Singapore have devised two tools to help chemists assess and optimise the chemoselectivity of N-heterocylic carbene (NHC)-mediated reactions on carbonyl compounds.
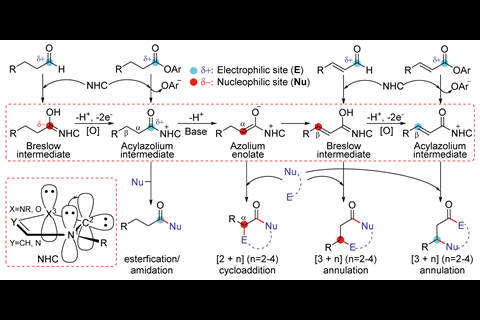
NHCs are powerful catalysts, used in a wide range of organocatalytic processes. Their reactions with carbonyl compounds lead to active intermediates like enolates, Breslow intermediates or acylazoliums. Predicting these intermediates and the final products of these reactions is very valuable.
However, when external bases or oxidants are added to the system, these can reverse the electronic properties of the usual intermediates. This makes it much more difficult to predict the reaction mechanism, and hence the products.
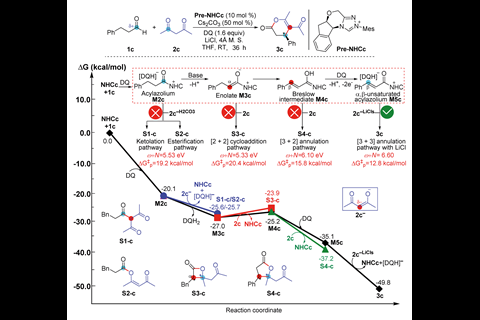
Now, a team led by Donghui Wei and Yu Lan of Zhengzhou University has studied various NHC-catalysed reactions and mapped possible pathways for reactions with bases and oxidants, exactly predicting the energy barrier of the chemoselective step. The complexity of such reactions is reflected in the map of possible intermediates for an NHC-catalysed reaction of an aldehyde with an acetylacetone. Their calculations enable the likely intermediate to be identified as the α,β-unsaturated acylazolium, leading to a [3+3] annulation.
The group also proposes a novel index to compute the activity and stability of the intermediates. This index comprises ω for the electrophilic component and N for the nucleophilic component. They demonstrate a linear relationship between the energy barriers of chemoselective transition states and the corresponding ω+N values of the reaction partners.
These tools will help chemists identify the active intermediates in some NHC-catalysed reactions, outline possible pathways and perhaps even the main products, significantly reducing the number of computations required when studying these nucleophilic and electrophilic reactions.









No comments yet