Click chemistry reaction can be reversed ‘without a trace’
Reactions that cleanly ‘click’ together are important for attaching useful groups to biological molecules, but have been difficult to undo – until now. Eric Anslyn’s team at the University of Texas at Austin in the US has developed a click chemistry system that unclicks on addition of a trigger compound.
‘The trigger allows you to add and then take a chemical label off again, without leaving a trace,’ says Katharine Diehl, now a postdoctoral researcher at Princeton University. Among various demonstrations, the Austin researchers have reversibly joined proteins and polyethylene glycol (PEG) chains, which are commonly used to improve drug properties.
The reactions also use water as a solvent, making them potentially ‘green’ and more likely to work inside living organisms. ‘We hope other people will have problems that we don’t know about that they’ll be able to solve using this concept,’ says Diehl.
During her PhD in Anslyn’s team, Diehl was originally seeking ways to synthesise large receptors that surrounded smaller template molecules using reversible reactions. She tried a wide range of ‘conjugate acceptor’ reagents, and stumbled on one whose reactivity could fulfil the specifications of click chemistry: simple, high yielding, without hard-to-remove by-products. Specifically, a molecule containing an amine functional group first couples to the conjugate acceptor, followed by a molecule containing a thiol. Anslyn, Diehl and their colleagues then realised that adding dithiothreitol (DTT) made the system unclick, triggering the original amine and thiol’s release.
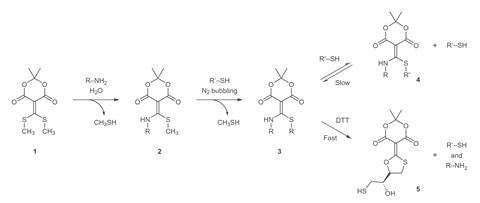
However, the reversibility of the process introduces limitations. Reactions must be done in water to avoid two amine molecules adding to the conjugate acceptor. Yet the conjugate acceptor itself is insoluble in water, so the Austin chemists add a little acetonitrile and accept some double addition. The ‘click’ is also slow, taking days for some amines and thiols. This is shortened by heating, but at the cost of by-product formation. ‘It’s definitely a practical challenge,’ Diehl admits. ‘That’s what you get with reversible chemistries – a balancing act – but optimisation for a particular system is possible.’
John Jewett at the University of Arizona, US, calls the method ‘a powerful new technique that enables chemical biologists to assemble, reassemble, and disassemble macromolecular structures at will’. ‘What makes this work shine to me is the thorough characterisation of the reaction and clear awareness and openness about possible limitations.’ The long reaction times and the need for DTT worry Jewett slightly, but he thinks both ‘can and will be addressed with future development’.















No comments yet