The disappearing polymorph of HIV drug ritonavir can now be recovered from its more stable nuisance form by a 15 minute ball milling procedure. Careful control of the milling conditions enabled researchers to manipulate the size and shape of ritonavir crystals to drive formation of the desired polymorph with complete selectivity. The method appears to solve a problem that caused chaos for drug manufacturer Abbott Laboratories in the late 1990s.
Polymorphism – the formation of multiple crystal forms for a single compound – is a huge challenge for the pharmaceutical industry. Different crystal structures exhibit different physical properties, which impacts the safety and efficacy of drugs. This makes controlling the crystal form vital.
‘Normally what crystallises first is what crystallises most easily – ie kinetically,’ explains Jeremy Sanders, a supramolecular chemist at the University of Cambridge, UK. ‘But that’s not necessarily the most stable form thermodynamically.’
This battle between kinetic and thermodynamic control gives rise to the phenomenon of disappearing polymorphs. If conditions spontaneously favour the formation of a thermodynamically more stable form, all the crystals will convert to the new structure and it becomes almost impossible to access the original kinetic product.
The 1990s ritonavir crisis that hit Abbott Laboratories (now AbbVie) is one of the most high-profile examples of this effect. ‘They formulated the drug on the knowledge of only one of the polymorphic forms, RVR-I,’ explains Aurora Cruz-Cabeza, a nucleation chemist at the University of Durham, UK. ‘Then a new polymorph appeared and completely contaminated everything – all the RVR-I converted into RVR-II and they couldn’t go back.’ Ultimately Abbott had to withdraw the drug from the market and reformulate at an estimated cost of $250 million (£200 million).
Now, Cruz-Cabeza’s team has shown that grinding processes like ball milling, which can induce changes in polymorphs, can reverse the disappearance of RVR-I. ‘When you grow crystals from solution, they’re in the order of at least micrometres in size so the bulk is going to dictate the thermodynamic stability,’ she says. ‘When you’re milling, you’re working with steady-state sizes in the order of nanometres, so the surface plays a huge role.’
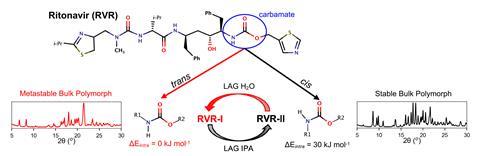
By moderating the grinding conditions (including time, solvent, and concentration), Cruz-Cabeza’s team controlled the size and shape of the crystals generated by the milling process, enabling them to switch between bulk-driven stability and surface-driven stability.
This interconversion is partly driven by the conformation of ritonavir’s carbamate bond. ‘The kinetic form (RVR-I) has its carbamate bond in the thermodynamically most stable trans conformation and crystallises easily from solution,’ says Sanders. ‘The cis conformation is thermodynamically less stable in individual molecules but turns out to make a more stable crystal – the thermodynamic form RVR-II.’
The bulk stability of large crystals favours RVR-II. Conversely, RVR-I forms preferentially where the individual molecular interactions at the surface of small crystals dominate the stability. Crystal shape also influences this surface energy and supporting computations by Cruz-Cabeza’s team showed that the energy overlap required for the interconversion only exists for needle-type crystals.
‘It’s a very rigorous piece of work. This theoretical approach, looking not just at the size or individual surfaces, but also the shape of the particles is quite innovative,’ says Linda Seton, a pharmaceutical chemist at Liverpool John Moores University, UK. ‘It would be interesting to see if this could be replicated for other systems. Milling could be used to search for new polymorphs and to enhance polymorph screening at an early stage.’
‘We’re really excited by these findings and this establishes that mechanochemistry is a unique tool for the discovery, recovery, and control of different polymorphic forms,’ says Cruz-Cabeza. ‘We’d like to understand the amorphous third phase, which must play a key role in facilitating the conformational conversion and linking this to computational methods to predict crystal structures is the next step.’
References
P Sacchi et al, PNAS, 2024, DOI: 10.1073/pnas.2319127121
















No comments yet