A new cross-coupling technique promises to unlock the unexplored chemical space of alcohols by expanding their use as building blocks in the synthesis of new and complex chemicals. The approach, which forms a carbon–carbon bond between two distinct alcohol units in one simple step, could also help reduce the time and resources usually required to access synthetic targets, including drug candidates.
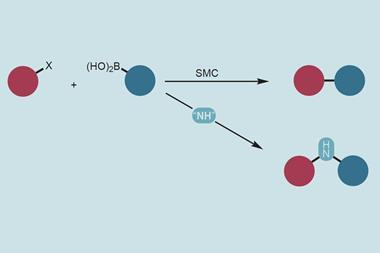
Alcohols are inexpensive and abundant with a lot of structural diversity. However, harnessing them for carbon–carbon bond-forming reactions – the core of organic synthesis – has remained a long-standing challenge. This is largely due to their strong carbon–oxygen bond, which is hard to break and thus makes activating alcohols particularly difficult, often requiring time- and resource-consuming pre-activation steps.
However, in 2021, David MacMillan’s lab at Princeton University in New Jersey, US, discovered a quick way to activate a single alcohol building block and break its C–O bond. This involved using N-heterocyclic carbene (NHC) salts and a light-triggered catalyst, which ultimately converted the alcohol unit into a transient carbon-centred alkyl radical that could then form new bonds with other functional groups.
Now, MacMillan’s team has applied this concept to simultaneously activate two distinct alcohol units in the same flask and shown how their respective radicals can be cross-coupled to form a new C–C bond. ‘Once we had identified the optimal conditions and began pushing the limits on scope, we knew we had something special,’ explains study co-author Nick Intermaggio. ‘The reaction setup is surprisingly easy and amenable to practitioners. We just have to mix necessary reagents one by one and then we get productive results within an hour upon shining blue light.’
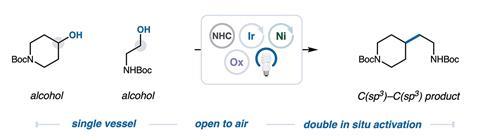
Key to the success of the C–C bond forming reaction was preventing the alkyl radicals from the two distinct alcohols coupling with their own kind and creating a chaotic and hard-to-control mixture of products. The researchers overcame this by incorporating a ‘radical sorting’ mechanism that they first described in 2022. This involved adding a nickel catalyst to the reaction, which forms metal–carbon bonds of different strength and reactivity with the two types of radical, ultimately ensuring that each kind of radical selectively reacts and forms a C–C bond with the other.
‘The concept of taking two different alcohols, clipping the oxygen atoms off and joining the carbon skeletons together is so simple on paper, but so incredibly hard to achieve in practice,’ comments Liam Ball, a synthetic chemist at the University of Nottingham, UK. ‘What the team has achieved is phenomenal and promises to radically change the way that organic chemists approach the construction of complex aliphatic architectures.’

Stephen Newman who researches alcohol cross-coupling at the University of Ottowa, Canada, is similarly impressed. ‘While the reaction is not perfectly selective and the yields throughout are usually modest, there’s no doubt that this is an incredibly efficient and exciting way of combining two attractive starting materials,’ he says. ‘Coupling two alcohols to make a new C(sp3)–C(sp3) bond should be very high on any synthetic chemist’s wish list, in particular for medicinal chemists who want to take abundant building blocks and rapidly make useful, complex, and structurally novel products.’
‘We hope that people will use this reaction to make complex structures that would otherwise be very difficult or take a long time to make. We already have folks from the pharmaceutical industry and total synthesis groups contacting us and wanting to use this technology to simplify their routes, which makes us very excited,’ says MacMillan. ‘All the reagents are commercially available and the reaction set up is very robust so once someone wants to use it, there should be little barrier to entry.’
References
R Chen et al, Science, 2024, DOI: 10.1126/science.adl5890







No comments yet