The recent outbreak of vaping-related deaths and illnesses in the US could be down to the pyrolysis, or thermal decomposition, of a vitamin E additive present in some unregulated vaping mixtures, according to researchers in Ireland. The team discovered the process can create an extremely reactive organic compound whose effects resemble those of a first world war chemical weapon.
The pyrolysis of phenyl acetate produces a highly toxic gas, so it occurred to me that vitamin E acetate was likely to have similar chemical reactivity
Donal O’Shea, Royal College of Surgeons in Ireland
Since September 2019, the Centers for Disease Control and Prevention (CDC) has recorded 57 deaths and over 2600 lung-injury cases related to vaping in the US in connection with unregulated vaping mixtures that contain tetrahydrocannabinol (THC) and cannabidiol (CBD) oils. Recent research has found that vaping-related health impacts were being reported as long as seven years ago. The first preliminary reports into solving the mysterious vaping deaths and illnesses suggested that a build-up of lipids or oils in the lungs could be responsible.
Vitamin E acetate, which has a honey-like consistency, soon fell under suspicion as the chemical culprit because it was known to be used as a thickening or diluting agent in many THC and CBD formulations. It’s considered non-toxic and used in vitamin supplements and skin creams, but inhaling it could cause health problems.
As the mystery unfolded, however, a further study in October led by Brandon Larsen at the Mayo Clinic in Scottsdale, Arizona, analysed 17 lung biopsies of patients with vaping-related injuries. His team found no evidence to support the idea that oil accumulation in the lungs was the underlying cause. But the team did see signs of chemical burns similar to those found after exposure to toxic chemical fumes.2
Chemical mystery
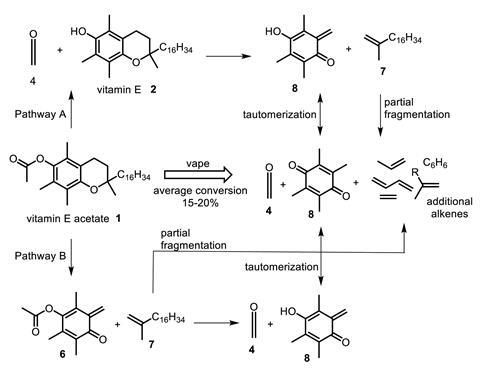
Intrigued by the reports, chemists Dan Wu and Donal O’Shea at the Royal College of Surgeons in Ireland have shown how vitamin E acetate could indeed be responsible for vaping-related deaths and injuries in a preprint that is currently being peer reviewed. When the molecule undergoes pyrolysis in a vaping device it creates a toxic gas that would explain death, lung injury and chemical burns.
‘I was aware that the pyrolysis of phenyl acetate produces a highly toxic gas, so it occurred to me that vitamin E acetate was likely to have similar chemical reactivity,’ explains O’Shea. ‘I ordered some vitamin E acetate and the first mass spectra we ran indicated that we were on the right track.’
Using mass spectrometry, computational calculations and trapping experiments with a vaping device – although in conditions that do not exactly replicate a user’s experience – the team found that vitamin E acetate was transformed during pyrolysis to produce ketene. Toxicity studies in primates have shown that inhaling ketene has a similar mode of action and is as harmful as inhaling the industrial chemical phosgene, notorious for its use as a chemical weapon in the first world war. Tests also revealed the presence of carcinogenic alkenes and benzene that are found in regular tobacco smoke.
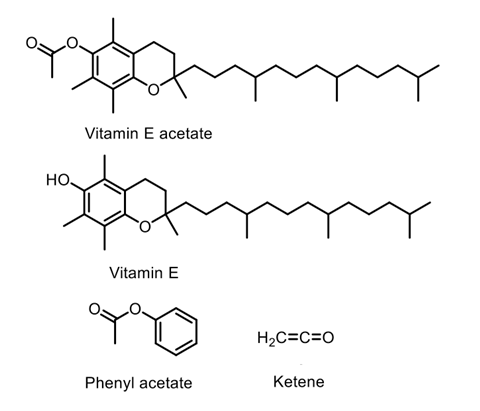
Ketene is a colourless, toxic gas with a sharp, penetrating odour, and at high levels is particularly nasty. It severely irritates the eyes and skin and if breathed in can cause serious damage to the lungs, which can be delayed for up to 24 hours after exposure. Animal studies with primates have shown that the minimum lethal in-air concentration is 200ppm, causing death after a single 10 minute exposure.
Further evidence
‘These findings are intriguing and offer a tantalising glimpse into the problem,’ comments Larsen, senior author of the previous lung biopsy study at the Mayo Clinic. ‘Our study suggested a direct toxic injury to the lung, akin to a chemical burn. This new work offers a potential explanation that bridges this gap. It is certainly possible that toxic byproducts from pyrolysis of vitamin E acetate, such as ketene, could be causing this injury, at least in some cases.’
Last month, the CDC confirmed the presence of vitamin E acetate in biological specimens obtained from patients with vaping-related lung injuries. While its presence alone does not confirm a causal link to lung injury, it adds further evidence to the role of vitamin E acetate in vaping-related illness. Further research by another team concluded that in 51 cases of probable or confirmed injury in vapers, vitamin E acetate was found in lung fluid samples of 94% of patients.3
However, O’Shea warns that the implications of his latest work are broader than vitamin E acetate alone. ‘The temperatures obtainable within vaping devices places them in the category of a small-scale laboratory pyrolysis apparatus which, if not used with precision and care, could have unforeseen outcomes,’ he says. ‘The pyrolysis products obtainable from other components of vape mixtures including flavours and additives also require investigation as they too may produce toxic and carcinogenic substances.’
References
1 D Wu and D F O’Shea, ChemRxiv, 2019, DOI: 10.26434/chemrxiv.10058168.v2
2 Y M Butt et al, N. Engl. J. Med., 2019, 381, 1780 (DOI: 10.1056/nejmc1913069)
3 B C Blount et al, New Eng. J. Med, 2019, DOI: 10.1056/NEJMoa1916433












1 Reader's comment