A group of researchers based in the US and Korea have developed and tested an edible matrix code made of silk that can be attached to tablets or added to liquids as an anticounterfeiting measure. The tag is made of silk and is invisible to the eye, but can be picked up by specific optical filters on a smartphone camera that can pick up fluorescence.
Drug counterfeiting is an increasing problem. Every year it causes thousands of deaths and poisonings, and recent increases in online sales have only made the problem harder to manage.
‘There are approximately 40,000 online pharmacies that one can access via the Internet. Only three to four percent of them are operated legally,’ explains Young Kim, a researcher at Purdue University who co-led the research.
Most anti-counterfeit measures are applied to medicine boxes, but some other ‘on-dose’ tags do exist, such as DNA tags. The problem with these is they mostly require skilled personnel and expensive machinery to process.
Kim and colleagues previously developed transgenic silk worms in South Korea that produce silk proteins in three distinct fluorescing colours to create anti-counterfeit tags. In the new study, they tested the technology more broadly, including adding tags to tablets and high-alcohol liquids, and training a smart phone application to read them.
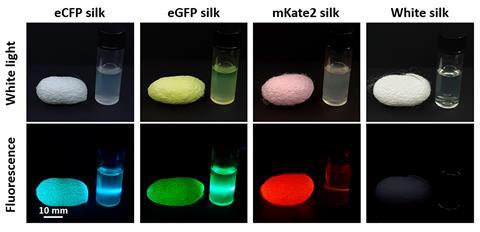
Several factors need to be addressed before the system can be rolled out, however. ‘We need to scale up in terms of production,’ says Kim, who is currently discussing the best way to do this with several pharmaceutical companies. He says estimated material costs are low, at 1-2 cents per pack, but adds that future labour costs are harder to estimate.
A possible scale-up approach includes adding tags directly during manufacturing. ‘That’s definitely the best case,’ says Kim, but adds that some kind of ‘sticker’ including the tag could also be attached to the medicines.
The ingestible nature of the tags, means FDA approval will have to be sought. But Kim is confident that this will not be a problem. ‘The good thing is silk proteins are now “generally recognised as safe”,’ he explains, an official FDA designation for non-toxic food materials.
Natalja Genina, a pharmacist at the University of Copenhagen focused on anticounterfeiting materials, applauds the innovative nature of the technology. However, she says ‘making sure that they can generate and store the data, and that everyone has access to this data, could be a challenge’.
Tim Mackey, a professor at the University of California, San Diego specialising in drug counterfeiting research, says lack of knowledge could also be a problem. ‘A lot of consumers aren’t aware of the counterfeiting medicines issue,’ he explains.
Kim concedes that more work is needed before patients can directly check their own medicines. Initially he and his colleagues plan to work with health care professionals to trial the anti-counterfeiting technology further.
An additional proposed application of the technology is monitoring patient adherence in clinical trials. The team also thinks the tags could help monitor counterfeiting of drinks such as whisky, as a high alcohol content is needed for the tags to maintain structural integrity.
References
J W Leem et al, ACS Cent. Sci., 2022, DOI: 10.1021/acscentsci.1c01233
















No comments yet