Elusive pyridine-based chloronium structures revealed
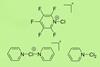
Researchers complete set of halonium ions by isolating a reactive chloronium structure
For the first time, researchers have reported the solid-state structure of pyridine-based chloronium ions. Previously reported to be intermediates in the oxidation of secondary alcohols, these nitrogen-ligated chloronium ions had proven challenging to isolate.