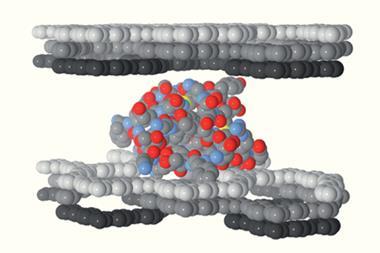
Four-disulfide insulin has similar bioactivity to native human insulin
Scientists in the US have made insulin more thermally stable, yet just as potent, by adding an extra disulfide bond. The insulin analogue could be beneficial for diabetes patients who struggle to access refrigerators to store their medication.
With an estimated 415 million people suffering from diabetes worldwide, many researchers have attempted to tweak the structure of insulin and improve treatment options for patients. Some of these have been successful and there are now insulin analogues that have fewer side effects, are faster-acting and require fewer injections.
Despite this progress, patients must store insulin in the fridge to avoid the proteins aggregating and inactivating. Previous attempts to create insulin with higher stability have simultaneously decreased its bioactivity. Now, an insulin analogue, developed by a team led by Christopher Hill and Danny Chou from the University of Utah, has shown increased thermal stability and similar in vivo potency in mice compared to native insulin.
Three disulfide bonds link two chains (A and B) in native human insulin. Hill and Chou’s team were inspired by the structure of an insulin venom, used by fish-hunting cone snails to induce hyperglycaemia and immobilise their prey, to find an appropriate region for a fourth disulfide bond. Native human insulin comprises an A chain of 21 amino acid residues (A1–A21) and a B chain with 30 residues (B1–B30), whereas the snail venom insulins have an extended A chain with 24 residues and a truncated B chain with only 22 residues.
The scientists extended the A chain of native human insulin and inserted an additional disulfide bond between the extended A-chain and B-chain residues B21–B23 and found success with residues A22 and B22. ‘This work discovered a novel and unexpected site to introduce an additional disulfide bond on insulin, which increases the overall stability of the protein,’ says Chou.
Songnian Lin, of Merck Research Laboratories, US, whose research explores glucose-responsive insulins, says that the work is very exciting. ‘The discovery by Chou and colleagues is a significant development in this area, with markedly improved aggregation stability while retaining the potency and structural features of the native insulin. This could be critical for manageable dosing volume, keeping an appropriate insulin pathway activation, and minimising potential mitogenicity and immunogenicity issues.’
While the insulin derivative is in the early stages of drug development, the result also presents further opportunities for research. ‘Our results demonstrated that tethering between the C-terminal A chain and the B21–B23 region can lead to bioactivity variation ranging from retained potency to 50-fold reduction,’ adds Chou. ‘This result presents an opportunity to control insulin bioactivity using this tethering strategy. For example, one may insert a glucose-responsive tethering here to achieve glucose-responsive insulin -– a holy grail in insulin therapy.’










No comments yet