Meera Senthilingam
This week, we clarify the importance of the often misunderstood molybdenum. Here's Quentin Cooper:
Quentin Cooper
The answer to the ultimate question – of life, the Universe and Everything – is, as every Douglas Adams fan knows, 42. And 42, as every Mendeleev fan knows, is the atomic number of molybdenum. And for many that – plus the indisputable fact that molybdenum is a funny word – is often about as far as their knowledge goes of this silvery metal – not that they'd have known it was a silvery metal – which is wedged between its better known brethren chromium and tungsten in group six of the periodic table. That odd-sounding name comes in a convoluted way from the Greek for lead, as ores of the two were often mixed up by early mineralogists – it was also frequently mistaken for graphite – and it wasn't until 1778 that molybdenum was recognised as a distinct entity deserving its own place in the periodic table, and a few years later still that it was finally isolated. The key breakthrough came from the Swedish chemist Carl Wilhlelm Scheele, better known as 'Hard luck Scheele' because he made a whole series of chemical discoveries, including oxygen, only for others to go and get the credit.

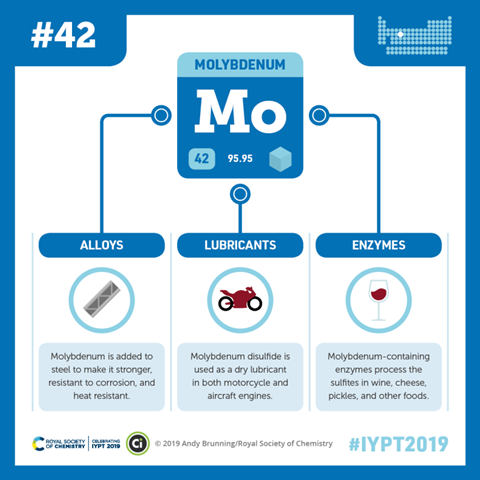
So its mistaken identity history, its miscredited discoverer, its misleading and often mis-spelled name, all add to the aura of comedy and confusion around molybdenum...and yet it's an element that's right at the root of life – not just human life, but pretty much all life on the planet: yes you'll find tiny amounts of it in everything from the filaments of electric heaters to missiles to protective coatings in boilers, and its high performance at high temperatures mean it has a range of commercial applications: it's useful in toughening up steel and giving it more corrosion resistance, as a catalyst in processes such as refining petroleum, and above all it's turned to when you need things to get hot but stay slippy – where WD40 and other petroleum derived oils are at risk of igniting, molybdenum sulfides are the basis of a range of lubricants which can cope with the heat and keep things moving smoothly.
But for all the ways we've discovered to use it, of far greater significance – although involving far smaller quantities of molybdenum – is the way we've evolved to make use of it within us. It's found in dozens of enzymes...including all important nitrogenase, which allows the most abundant element in the atmosphere, nitrogen, to be taken up and turned into compounds that enable bacteria, plants, us and everything between to synthesise and utilise proteins. Without proteins there wouldn't be much at all in the way of life...and without molybdenum there wouldn't be much at all in the way of proteins. And it turns up in other key human enzymes too such as xanthine oxidase in the liver, which is vital to our waste processing.
But just in case anyone's thinking of rushing off to buy one of the many commercially available trace mineral supplements with molybdenum it's worth adding that although like much of life on Earth we definitely need it...we don't need that much of it: about a third of a gram is all you'll get through in an entire lifetime. That's next to nothing...but without it we'd be next to nothingness.
So, time to stop laughing at the funny name...molybdenum really is one of life's few true essentials.
Meera Senthilingham
So time to give some much-owed respect, it seems, to the element molybdenum. That was science broadcaster Quentin Cooper with the widely applied chemistry of molybdenum. Now, next week, blink and you may miss it.
Brian Clegg
If elements were insects, darmstadtium would be the mayfly of the chemical world. It exists for the most fleeting time before it transforms to something else. Darmstadium is never going to have a practical use – but its sheer brevity of existence gives it a wistful fascination.
Meera Senthilingham
And to find out what does happen in darmstadtium's brief existence on earth, in next week's Chemistry in its element. Until then, I'm Meera Senthilingham and thank you for listening.













No comments yet