New research on how psychedelic drugs promote neuron growth in the brain has thrown light on the therapeutic effects these drugs display in the treatment of mental health conditions. The work helps to unravel why some drugs that bind to serotonin receptors bring sustained antidepressant effects that other compounds, including serotonin itself, don’t produce.
Psychedelic drugs like psilocybin – the key active ingredient in magic mushrooms – have been shown to offer benefits in treating mental health conditions like depression. Psilocybin was officially recognised as a medicine for the first time in a decision by Australia’s Therapeutic Goods Administration earlier this month.
One of the main reasons that psychedelics are believed to have therapeutic effects, is that they are known to promote plasticity in cortical neurons. This means that the drugs encourage neurons to grow new branches and make more synaptic connections – a key feature of a healthy brain.
Now, researchers in the US have shown that psychedelic drugs promote this neuronal growth by activating intracellular serotonin receptors that are actually inaccessible to serotonin itself. David Olson, the director of the Institute for Psychedelics and Neurotherapeutics at the University of California, Davis, explains that the project stemmed from a problem that had puzzled his group for a long time.
Serotonin puzzle
‘We did a lot of mechanistic work that demonstrated that the serotonin 2A receptor is responsible for the plasticity promoting properties of psychedelics in the cortex, and some of their therapeutic behavioural effects,’ says Olson. ‘But what really surprised us was that serotonin itself did not have a lot of these same properties.’
This observation bothered Olson because, as he puts it, ‘serotonin is a very good agonist for the serotonin 2A receptor’. But serotonin only activates receptors on the outside of a neuron.
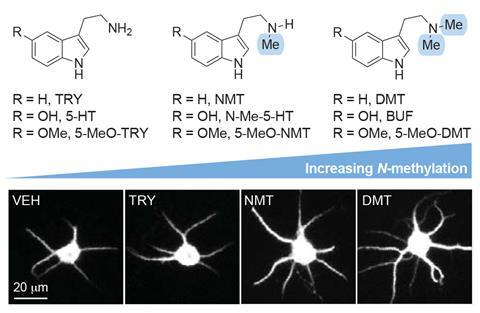
So, Olson’s team set about investigating the structure–activity relationship of compounds that bind to serotonin receptors. By making simple modifications to serotonin and related compounds, these experiments revealed that the molecules’ lipophilicity – their greasiness – seemed to correlate with their ability to promote neuron growth. ‘That suggested to us for the first time that maybe the target was on the inside of the cell,’ says Olson. ‘And so these really greasy compounds that can cross cell membranes can access the target, but very polar molecules like serotonin might not be able to.’
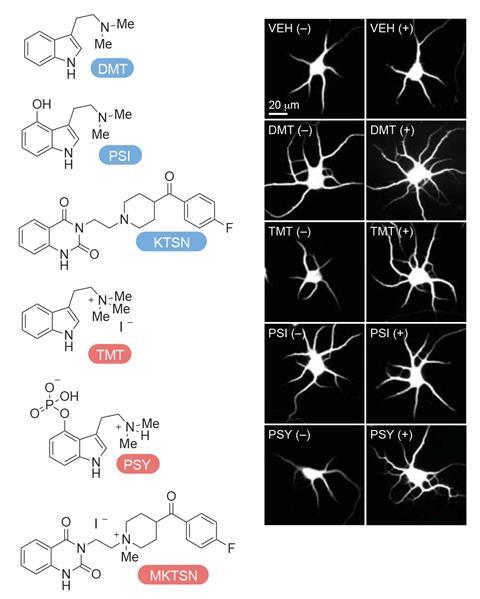
The researchers then chemically modified compounds that would usually activate intracellular serotonin receptors so that they could no longer cross cell membranes. These experiments showed that charged versions of molecules like psilocybin and DMT – a psychedelic drug that is also produced naturally in small quantities in mammals – could no longer promote cell growth under normal conditions. They then used a technique called electroporation, where a voltage is used to temporarily open up pores in the cell membrane, allowing the charged molecules to cross into the cell.
‘And when we do that, we see something very different,’ says Olson. ‘Now, the membrane-impermeable agonists can promote growth and the membrane-impermeable antagonists can block growth – again suggesting that the target is on the inside of the cell.’
The team further underlined the importance of activating intracellular receptors by showing that neurons that express a protein capable of importing serotonin across the cell membrane also demonstrate improved plasticity when treated with serotonin. They then showed how this effect could be harnessed to increase the uptake of serotonin in cortical neurons in live mice, leading to measurable antidepressant effects.
Location, location, location
Mark von Zastrow, an expert on cellular signalling machinery based at the University of California, San Francisco, who was not involved in the project, says that the findings extend the concept of location bias – the idea that G-protein coupled receptors can produce different physiological signalling effects depending on the location within a cell at which they are activated.
He notes that Olson’s team’s findings that structural plasticity is linked to the cellular location of activated serotonin receptors could help in the design of new therapies to treat mental health conditions. ‘This discovery suggests a new therapeutic strategy for the treatment of depression by developing drugs which selectively activate signalling from internal receptors,’ he says.
Another question raised by Olson’s team’s project is whether serotonin is really the natural ligand for intracellular serotonin receptors inside cortical neurons. ‘One possibility is that perhaps there are ligands that can cross membranes and activate these receptors,’ says Olson.
He suggests that an ‘obvious candidate’ would be endogenous psychedelics. These are psychedelic drug-like compounds, like DMT, that are produced naturally in mammals, and whose purpose remains something of a mystery. According to Olson, it’s possible that these molecules might play a more important biological role than is currently realised.
‘While we know that they exist, we don’t really know if they’re functionally relevant at this point in time,’ he says. ‘But the fact that the receptor is held out of reach from serotonin itself suggests that maybe they play a role at some point in normal physiology.’
References
M V Vargas et al, Science, 2023, DOI: 10.1126/science.adf0435













No comments yet