A team of researchers in the UK has developed a new way to upcycle PTFE, using only lumps of sodium metal and mechanical force. This simple method creates a source of fluorine which can be used to synthesise a range of valuable fluorinated small molecules.
Polytetrafluoroethylene (PTFE) – more commonly referred to by its brand name Teflon – is known for its thermal and chemical resistance, owing to it’s hard to break carbon–fluorine bonds. Such attributes make it an ideal material for lining reaction vessels, batteries and non-stick frying pans.
‘The properties that make [PTFE] so amazing also creates a big problem when it comes to disposing of it,’ says Roly Armstrong at Newcastle University in the UK. He adds that traditional disposal methods are limited to either sending it to landfill, or incineration, both of which often create smaller chain per- or polyfluoroalkyl substances (PFAS) that can build up in the environment.
Researchers have now started looking at other ways of disposing of waste PTFE – among other fluorinated polymers – with an increased focus on upcycling such materials into useful fluoro–containing reagents and molecules. Recent advances by Véronique Gouverneur and her group at the University of Oxford, UK earlier this year involved mechanochemically breaking down PTFE into fluorinated phosphate salts.

Armstrong is a part of a collaboration between Newcastle University and the University of Birmingham, UK, that has now developed a method that instead uses cheap and readily available sodium metal as the reagent. ‘We take the Teflon and sodium metal [under an inert atmosphere], put them in a stainless steel jar with ball bearings and shake it at a high speed of 30Hz for one hour,’ explains Dominik Kubicki at the University of Birmingham, adding that there is no need for any solvents or further processing.
Ina Vollmer, an expert in chemical upcycling at Utrecht University in the Netherlands, says that ‘this method seems much simpler than previous studies, while also achieving a single product’. Spectroscopic analysis revealed that the only products are highly-pure sodium fluoride and elemental carbon.
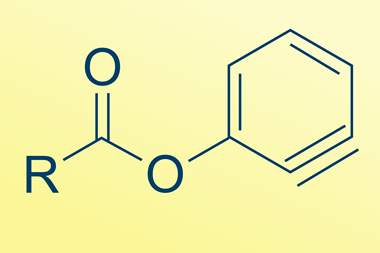
The UK-based team were able to use the sodium fluoride powder without purification to fluorinate a range of sulfonyl and acyl chlorides, using the same mechanochemical method as used to initially upcycle the PTFE. Armstrong explains that such acyl and sulfonyl fluoride products are ‘very important in lots of synthetic transformations’.
‘The field is rapidly shifting from fluoropolymer destruction towards true fluorine resource recovery,’ says Norio Shibata, a fluorine chemist at the Nagoya Institute of Technology, Japan. ‘This [work] provides valuable momentum to that transformation.’
Moving forwards, the team are looking to scale up their current method and better understand the mechanism behind how sodium helps break down PTFE, as well as investigate the upcycling of other fluorochemicals using similar methods.
References
M E Lowe et al, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c14052





















No comments yet