Stereochemistry can be used to tune the density and detonation performance of cage-like energetic materials, new research shows.1 This research on three-dimensional structures builds on a small body of work on two-dimensional materials2,3 questioning previous assumptions that isomeric variations have little impact on energetic parameters.
‘Computational methods used to predict properties of energetic materials are usually done in the gas state and don’t consider stereochemistry. So, we thought it was important to synthesise and test some 3D compounds, which arguably should be more affected by stereochemistry compared to 2D compounds that usually have lower melting points and are sometimes even liquids,’ explains Jun Luo, at Nanjing University of Science and Technology in China.
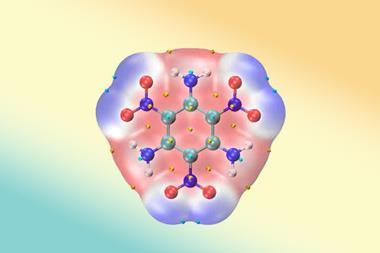
Luo’s team used a 2,4,10-trioxaadamantane backbone to synthesise two series of 3D energetic stereoisomers. They selected (exo,endo,endo)-2,4,10-trioxaadamantane-6,8,9-triol, which can be prepared from the commercially available natural product inositol, as the starting point due to its favourable molecular designability. Stereoselective peripheral editing of its three hydroxy groups by primarily exploiting the steric hindrance (or lack thereof) of protecting groups and reducing agents yielded four trinitrate ester diastereomers and three tetranitro diastereomers with relative ease.
Density is a critical parameter for energetic compounds, with a material’s detonation pressure being proportional to the square of its density.4 Single crystal x-ray diffraction analysis of the seven energetic compounds synthesised uncovered distinct differences in their crystal packing arrangements and thus density.
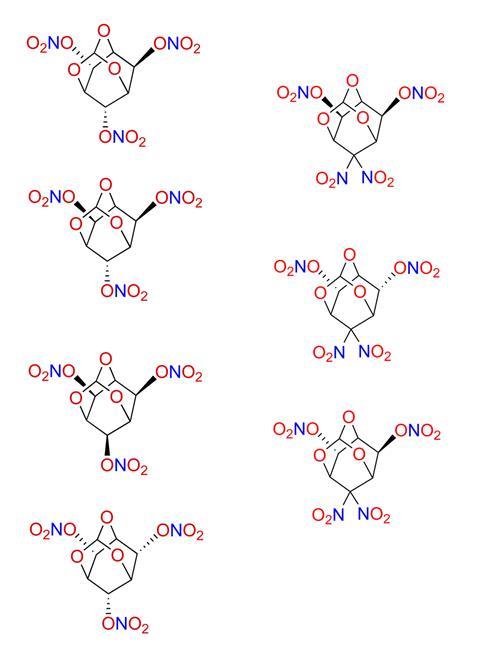
Of the seven compounds, the (exo,endo)-tetranitro isomer exhibited the highest crystal density at 1.980g cm⁻³, surpassing that of most currently used energetic compounds. ‘The density for this isomer was so high that we even measured a new batch of crystals at a different beamline to double check the results. It almost reaches the upper limits of energetic materials based on carbon, hydrogen, oxygen and nitrogen – only a handful have been reported with densities exceeding two,’ says Luo. The team attributed the higher-than-expected density of the (exo,endo)-tetranitro isomer to strong intermolecular hydrogen bonding.
Additionally, in the trinitrate series of isomers, all-exo and all-endo compounds exhibited higher crystal densities compared to their lower symmetry isomers. The team say these results showcase the promise of using configurational isomerism as an effective strategy for designing energetic materials with better properties.
A lead plate perforation test compared the highest density (exo,endo)-tetranitro compound to its lowest density diastereomer and the commercial explosive RDX. In this test, the diameter of the holes left in lead plates after detonation served as a measure of explosive performance. As expected, the high-density isomer outperformed its diastereomer and showed results comparable to RDX, reinforcing its potential as a viable secondary explosive.
‘This work will encourage energetic material chemists to re-examine existing molecules and encourage them to modify them further by simply changing the relative positions of the explosophores to enhance their energetic and physical properties further,’ comments Dheeraj Kumar, an expert on high energy density materials at the Indian Institute of Technology Roorkee. ‘It will also encourage hardcore organic chemistry researchers to contribute to the field of energetic materials, as such a type of structural modification requires deep knowledge of organic synthesis.’
References
1 H Li et al, Chem. Sci., 2025, 16, 15587 (DOI: 10.1039/d5sc02800k)
2 L M Barton et al, J. Am. Chem. Soc., 2019, 141, 12531 (DOI: 10.1021/jacs.9b06961)
3 K Rykaczewski at al, J. Am. Chem. Soc., 2022, 144, 19089 (DOI: 10.1021/jacs.2c08191)
4 M J Kamlet and C Dickinson, J. Chem. Phys., 1968, 48, 43 (DOI: 10.1063/1.1667939)











No comments yet