One of the most frustrating problems faced by synthetic chemists – crystallising a sample – could be solved by a new process that uses a robot to create hundreds of tiny crystallisation experiments. The technique maximises the chances of crystals forming, while using minimal quantities of material. Its developers have already used it to grow crystalline samples of a compound previously considered ‘uncrystallisable’.
When a new material is produced in crystalline form, its structure can be probed by x-ray crystallography – one of the most powerful tools for compound characterisation. But growing crystals can be difficult. It usually involves a trial-and-error approach of slowly drying solvated samples under different conditions, in the hope that crystals might form. Setting up the experiments takes time and often uses up a lot of material – of which there may be a limited supply.
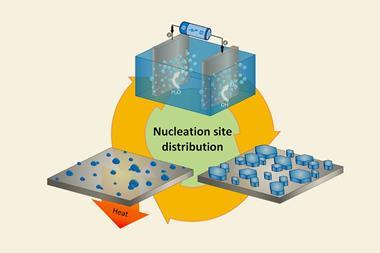
Now, a high-throughput approach to crystallisation could help ease the pain of obtaining crystals. The method, which uses micrograms of material dissolved in nanolitres of organic solvent, takes inspiration from ‘microbatch-under-oil’ techniques used by protein crystallographers to control the crystallisation process.
We’d be interested if anybody has something they’re struggling with and want to either have a go at it themselves, or let us have a go at it
Michael Hall, Newcastle University
‘We were aware of all the advances that have been made in protein crystallography, where you can grow crystals on a very small scale, you can automate a lot of the experimental work, and that’s led to huge advances in that field,’ says Michael Hall, who co-led the project with Michael Probert at Newcastle University. ‘So we put our thinking hats on to see if we could do something similar for small molecules.’
Hall’s team use a liquid handling robot to encase nanodroplets of sample solution inside larger droplets of oil. This promotes crystallisation by reducing the number of nucleation sites and allowing a slow increase in sample concentration, explains Hall. ‘If you’ve got a very small droplet of solvent, the evaporative loss is very fast. And if you just try that on a on a glass slide, for instance, you just get amorphous material depositing – it’s very hard to get anything crystalline,’ he says. ‘We thought if we could put a little droplet of organic solvent into a bigger droplet of oil we could control that rate of loss – because we should be able to limit the solvent–air contact of the organic solvent droplet.’
As the technique uses a robot to handle the samples, hundreds of crystallisation experiments can be set up within minutes. This increases the chances of finding the right conditions for crystals to grow.
57-year-old challenge
The team tested the system with a range of small molecules including organic dyes, organometallics and pharmaceuticals. Compared with traditional crystallisation methods, the encapsulated droplets were more likely to produce high quality crystals, and even led to the discovery of a new polymorph of a widely studied pharmaceutical intermediate – the precursor to the antipsychotic drug olanzapine.
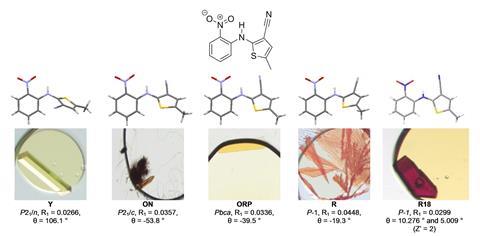
Having seen a high-success rate, the team then turned to a more difficult challenge. The fungicide dithianon was first synthesised in 1963, but since then no crystals of sufficient quality for single crystal x-ray diffraction had been reported. ‘Our industrial colleagues highlighted this molecule to us as a challenging case,’ says Hall, who explains that the compound’s weak intermolecular interactions had previously led to it being described as ‘uncrystallisable’.
The team set up 394 nanodroplet experiments using various mixtures of different oils and solvents. 72 of these produced crystalline samples.
The new technique is ‘an exciting addition’ to the crystallographer’s toolbox, says Louise Dawe, an expert on small molecule crystallography based at Wilfrid Laurier University in Waterloo, Canada. ‘Encapsulated nanodroplet crystallisation offers the advantage of working on a very small analyte scale, 1000 times smaller than traditional methods,’ she explains. ‘This minimises the amount of valuable synthetic product required for characterisation by single crystal x-ray diffraction, and requires only an extremely small amount of solvent, which offers green advantages by minimising waste.’ Dawe believes that the method ‘will be of significant industrial interest’, for example accelerating the search for new solid forms of pharmaceutical ingredients with beneficial properties.
That view is echoed by Susan Reutzel-Edens, an expert on crystallisation chemistry based at Eli Lilly’s small molecule design and development facility in Indianapolis, US. ‘Because it is not yet possible to predict a priori the experimental conditions to crystallise a molecule for the first time, in a particular crystal form, or in a specific size and shape, platforms to efficiently and effectively survey diverse crystallisation conditions are indispensable,’ says Reutzel-Edens. She adds that the technique ‘should find widespread application in both academic and industrial settings’.
Hall and his co-workers are in the process of setting up a spin-out company to help others make use of the technique, and have already been working with other academic groups to help crystallise samples that they have been struggling with. ‘We’d be interested if anybody has something they’re struggling with and want to either have a go at it themselves, or let us have a go at it,’ adds Hall. ‘We would be very keen to talk to people about this.’
Update: Susan Reutzel-Edens comment was added on 14 May 2020.
References
A R Tyler et al, Chem, 2020, DOI: 10.1016/j.chempr.2020.04.009








No comments yet