The habitat that cells live and grow in has been recreated using a mixture of self-assembling peptides and natural proteins.1 This synthetic matrix was then used to grow cancer spheroids, which could be used to test new anticancer treatments.
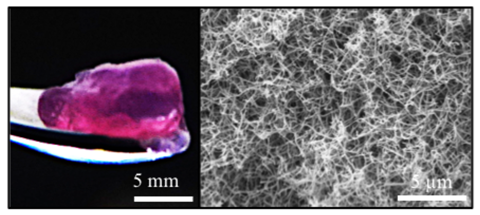
The process was extremely simple. A team led by Alvaro Mata, from the University of Nottingham, UK, mixed all the ingredients with the cells and watched the magic happen. ‘Self-assembling peptides have an amphiphilic structure, they quickly arrange themselves forming long fibres resembling those that hold cells together,’ explains Mata. The team used the exact same proteins found around natural tumours, such as keratin and fibronectin. ‘We used these peptides to co-assemble with proteins found in the natural tumour, creating a tuneable composite biomaterial that mimics molecular and structural features of the extracellular matrix,’ he adds.
The extracellular matrix that holds cells together is a complex environment, which provides structural support to the cells, and facilitates chemical communication between them. For years, researchers have bioengineered artificial scaffolds to replicate cell growth in vitro using synthetic polymers such as polyethylene glycol and small peptides. However, these solutions were poor mimics of the real thing. The new bioengineered nanofibres imitate the matrix much better.
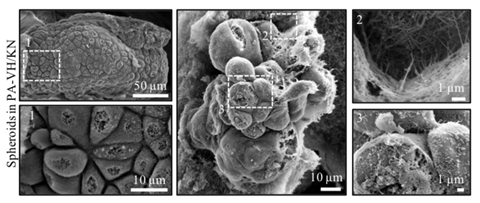
To test their model, the team cultured a range of different ovarian cancer cells. After a few days, they observed the formation of spheroids – microscopic structures that normally grow within tumours. ‘These spheroids exhibited behaviours like real cancer, progressively increasing their size,’ comments Daniela Loessner, from Monash University, Australia, who co-authored the study. However, when researchers added well-known antitumour agents – paclitaxel and carboplatin – the expansion stopped. ‘We see that drugs trigger remission, suggesting that our approach mimics real cancers, and potentially could be used to screen for new therapies in vitro,’ she adds.
Traditionally, researchers grew tumour cultures in Matrigel – a proprietary protein mixture isolated from mouse cancer cells. ‘Matrigel works really well, but its composition is unknown and fluctuates from batch-to-batch,’ explains Helena Azevedo, an expert in biomedical engineering from Queen Mary University of London, who was not involved in the study. ‘Moreover, it comes from mice, so it would be nice to find a synthetic alternative,’ she adds. Azevedo is also impressed that the researchers recreated the complexity of the extracellular matrix from scratch. ‘Knowing exactly what constitutes the extracellular matrix, we can determine and understand the most important factors in tumour development.’
Patricia Dankers, an expert in functional biomaterials from the Eindhoven University of Technology, Netherlands, is similarly impressed. ‘Culturing such a complex tumour model is a challenge,’ she says. Furthermore, the follow-up test with antitumour drugs proves the model is useful. Dankers is convinced that, in the future, ‘this very smart idea could replace Matrigel’. Tunability is key: ‘Extending the library of small molecules and natural proteins can lead to new hydrogels with complex compositions and different functions … adjusting to the needs of the cells.’ Unlike Matrigel, scaling-up production should be easy.
Further studies will determine the scope, efficiency and adaptability of these synthetic extracellular matrices. ‘Gradually, our aim is to build more realistic artificial tumours, enhancing how we test new chemotherapeutics,’ concludes Mata.
References
CL Hedegaard et al, Sci. Adv., 2020, DOI: 10.1126/sciadv.abb3298













1 Reader's comment