Claire Jarvis looks at ongoing work to prevent the disease – and convince a sceptical community of their seriousness
Many academics showcase press coverage of their research inside their offices, and Stephen Johnston of Arizona State University in the US is no exception. But the 2012 Nature editorial is a slightly unusual choice to pin to his door. ‘Misguided cancer goal,’ thunders the headline. ‘Hope is not a good strategy, in life or in disease research.’
The editorial takes aim at Johnston’s efforts towards creating a preventative cancer vaccine: a vaccine that, once administered, would stop certain types of cancer from growing within the recipient’s body. The public hostility and scepticism from the scientific community stems not only from the difficulty of accomplishing such a task, but is also a reflection of the high level of interest in using the body’s own immune system to defeat cancer.
The first thing to know about cancer vaccines is that most of them they aren’t vaccines – at least not in the sense that the general public usually thinks of them. Many current cancer vaccines are therapeutic, not preventative: they are administered after the patient is diagnosed to boost the body’s natural defences against cancer.
The second thing to know about cancer vaccines is that they aren’t a new concept. Researchers have been interested in preventing cancer for a long time, but the levels of optimism towards attaining such a goal have cycled as our understanding of immunology and cancer’s self-defence mechanisms has evolved.
Although unplanned, the Bacillus Calmette–Guérin (BCG) vaccine is the oldest anti-cancer vaccine. It was designed to treat tuberculosis, but as early as the 1920s physicians knew patients who received the vaccine were less likely to suffer bladder cancer. In 1990 it was approved by the FDA for the treatment of early-stage bladder cancer, though thanks to the decline of TB the BCG vaccine is no longer commonly administered in the west.
Almost every cancer patient has an immune response to their cancer
Vaccines against viruses that cause cancer are well-established. Chronic infections of hepatitis B and C can cause liver cancer. A hepatitis B vaccine was first approved in 1981. The human papilloma virus (HPV) vaccine was first approved in 2006. HPV is most commonly associated with cervical cancer, but the HPV vaccine has been found to decrease the incidences of anal and vaginal cancers, which are caused by different strains of HPV.
However, only 10% of cancers are viral in origin. Training the body to recognise and defeat cancer cells that spring up of their own accord is not so simple.
Checkpoint Charlie
In 2011 ipilimumab, the first immune checkpoint inhibitor drug, was approved by the FDA for treating metastatic melanoma. Immuno-oncology was now a viable cancer treatment option.
‘Over the last decade we’ve come to understand that almost every cancer patient has an immune response to their cancer,’ says Mary Disis, a cancer specialist at the University of Washington School of Medicine in Seattle, US. The problem is this natural immune response is to produce antibodies, which aren’t enough to destroy cancer cells by themselves. A stronger immune response (called a type 1 response) from the white blood cells called T-cells are needed to kill cancer cells.
In peacetime, the body’s immune checkpoints prevent the immune system from becoming over-aggressive and destroying healthy cells. When a T-cell comes into contact with another cell, it will attack unless it can bind with an immune checkpoint protein on the cell’s surface. Once bound via the immune checkpoint, the T-cell is deactivated. Two of the most common immune checkpoint proteins are PD-L1 and CTLA-4. The problem with cancer cells is that many of them have also immune checkpoint proteins on their surfaces, tricking the T-cells into viewing them as friend, not foe
Immune checkpoint inhibitors bind to either the proteins on the cancer cell or the T-cell side, preventing the two from coupling together. The T cells are no longer deactivated, so they destroy the cancer cells. The trouble with immunotherapy is it doesn’t always work. In fact, only 20–30% of patients will respond to immune checkpoint inhibitor drugs. As researchers elucidated the reasons for the unpredictable efficacy, they realised cancer vaccines could help bridge the responsiveness gap.
The problem is that tumour form their own immunosuppressive microenvironment body. ‘Once a cancer has developed to the point that it’s clinically detectable, it has already evolved a panoply of mechanisms and strategies to avoid detection by the immune system,’ says Doug Thamm, a professor of oncology at Colorado State University, US. Simply blocking the immune checkpoint from working is not enough.
We still don’t fully understand how and when cancer is initiated
‘For immune checkpoint therapy to work the patient also needs good T-cell immunity. So now the hypothesis is that one of the mechanisms to induce good T-cell immunity is vaccination. That’s why people are now going back to the vaccines, trying to see if we can induce good immunity in patients not responding to current immunotherapies.’ explains Periasamy Selvaraj, a professor of pathology at Emory University in Atlanta, US.
A personalised approach
Cancers mutate rapidly, creating variations unique to individual tumours. Instead of looking for common targets, many researchers therefore want to personalise their vaccines to the patients. ‘We still don’t fully understand how and when the cancer is initiated,’ says Lei Zheng, a professor of oncology at Johns Hopkins University in Baltimore, US, who argues that this lack of knowledge makes it harder to find universal ways to stop cancers from growing.
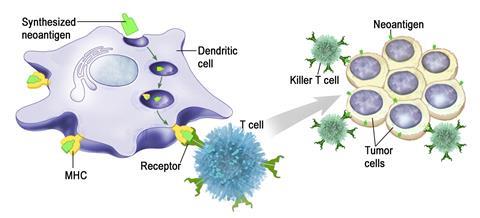
All vaccines need an antigen target. Antigens are substances which induce an immune response in the body, such as the production of antibodies. Neoantigens are newly formed antigens that have not been previously recognized by the immune system. Mutations in the tumour genes can cause them to produce mutated proteins that the body hasn’t seen before, making them a perfect target to vaccinate against.
To make a personalised cancer vaccine, the patient’s tumour genome is sequenced and all its mutations are identified. A computer program predicts likely neoantigens, and a vaccine expressing those neoantigens is manufactured. The process takes two to four weeks.
Currently Sipuleucel-T, marketed as Provenge by Valeant Pharmaceuticals, is the only approved therapeutic cancer vaccine on the US market. It was approved in 2010 for the treatment of advanced prostate cancer. As a treatment it only extends the patient’s life by a couple of months, and is used when the patient stops responding to other cancer therapies.
Another personalised cancer vaccine, Oncophage, has struggled to reach the market. The vaccine contains heat shock peptides, overexpressed in some tumours, which were extracted from the patient’s cancer cells. Antigenics’ application to market the drug (brand name Vitespen) in Europe was withdrawn in 2009, after they concluded the therapeutic benefits it provided to melanoma patients was limited. The vaccine was subsequently approved in Russia.
Things heat up
Making an effective cancer vaccine isn’t easy. A key challenge of therapeutic vaccines is getting T-cells into the tumour. Lei Zheng, Elizabeth Jaffee and other researchers at Johns Hopkins have developed GVAX with the aim of doing better for pancreatic cancer. The patient’s tumour cell line is genetically engineered to secrete a cytokine called GM-CSF, which encourages localised dendritic cell production. Dendritic cells act as relay messengers, collecting antigens from different parts of the body and bringing them to T-cells in the lymph nodes. When GVAX is injected, the dendritic cells produced at the cancer site will bring cancer-related antigens to the attention of the immune system.
Tumour cells taken from the patient are killed to ensure they don’t replicate. Once injected with the vaccine, the tumour turns from cold to hot. ‘This hot tumour starts to have robust infiltration of T-cells, which you wouldn’t usually see in pancreatic cancer,’ Zheng says .
GVAX was tested in conjunction with immune checkpoint inhibitors against pancreatic cancer. The vaccine showed promise, but in some clinical trials GVAX was no more effective than existing treatments at delaying cancer progression. Jaffee, Zheng and coworkers are also modifying GVAX slightly to target other kinds of cancer, such as colon and breast.
Sometimes what works in a mouse may not work in humans
Some cancers are less amenable to the personalised vaccination approach than others. Disis and her team are trying to immunise against breast, colon and ovarian cancers. These solid tumours have low mutation rates – they don’t produce many mutated proteins, so the immune system doesn’t see them as foreign threat.
Disis looked at the full protein sequence of these non-mutated antigens and found there are segments of the protein visible to the immune system that preferentially stimulate a type 1 or type 2 responses. ‘You can chop off parts of the non-mutated proteins and make them vastly more immunogenic,’ explains Disis. Removing the parts of the protein that stimulated a type 2 response and leaving all the type 1 segments produced a stronger immune response. Her team has bundled multiple abbreviated antigens into a single vaccine and given it to breast cancer survivors in the hopes it will prevent reoccurrence. Several phase I clinical trials are using these vaccines, and a phase II clinical trial for HER2-positive breast cancer patients is ongoing.
Another challenge of personalised cancer vaccines is that heterogeneity exists within the tumours themselves. ‘When you try to develop a cell line in vitro, most of the time only a few colonies grow,’ says Selvaraj. ‘For example, if there are 10 different clones in the cancer of a person, only one or two will grow in vitro.’ This means there’s a risk of your personalised cancer vaccine targeting only a small proportion of the tumour cells.
To circumvent this issue, Selvaraj and his team don’t culture the patient tumour cells. Instead they excise tumour membrane vesicles; the membranes themselves contain a high concentration of surface proteins the immune system could recognise as antigens. Cytokines, antibodies and lipids are clipped on to the vesicle material through glycophospate linkages before everything is injected back into the patient. This combination of antigens clumped together with several pieces of the immune response toolkit help direct a strong immune response to the cancer site.
The vaccine Selvaraj has developed is being tested against aggressive forms of breast cancer. The company he founded, Metaclipse Therapeutics, has tested this approach in animal models, and is preparing to request clearance for a Phase 1 clinical trial. One limitation of this approach is that it requires a lot of tumour tissue. Metaclipse Therapeutics’ approach needs to get at least one gram of tumour tissue from a patient. Not all tumours are that large.
Early laboratory studies are promising, though it’s not clear how the results in animal models will translate into humans. ‘Humans are highly unique species. Sometimes what is working in a mouse may not work in humans; sometimes it’s working in mice but when we go to humans it works much better,’ Selvaraj explains.
(Moon)shot in the dark
In choosing to hunt for a preventative cancer vaccine, Johnston is taking the less-trodden research path. Crucially, he wants to stay away from personalised vaccines: ‘They’re expensive, they take a long time to formulate, they’re complicated. Personal vaccines would never be affordable to the majority of people who get cancer.’
For a preventative cancer vaccine to work it needs to go after a target marker that’s unique to the cancer, and is conserved across a large patient population. The reason we don’t yet have an approved preventative cancer vaccine is because it’s very hard to find such a target.
Johnston and his team decided to focus on mutations in the cancer cell’s RNA. ‘The field has all focussed on mutations in the DNA, which occurs about a thousand times less frequently than it does in the RNA,’ says Johnston. After examining cancer tissue from 800 patients, they found several thousand conserved RNA frameshift mutations that could form the basis of a general vaccine. He’s unsure why other researchers haven’t used RNA in cancer therapies before, beyond it being a collective blind spot for the field.
The other challenge of developing a preventative cancer vaccine are the logistics involved in proving it works. Monitoring a large clinical trial population to prove the vaccine leads to decreased prevalence of cancer over the human lifespan would be too expensive and slow to be viable.

Here, Johnston found another workaround. Those 800 cancer patients his team screened weren’t human patients. ‘The average dog gets cancer when they’re 7–9 years old. The average person gets cancer when they’re 50–70 years old. That old adage that seven human years is equivalent to one year in dogs is not far off from being accurate,’ explains Thamm, who collaborated with Johnston at the clinical end of the preventative cancer vaccine project.
Canines are a reliable animal model for testing human cancer treatments. Dogs are exposed to the same environments as humans, so tend to develop similar cancers. Canine cancers come up naturally as dogs age; they aren’t induced in a laboratory setting as they are with rodents. This gives researchers the opportunity to run accelerated clinical trials, since dogs have shorter lifespans.
The vaccine they’ve created contains antigens of the eight most commonly occurring canine cancers. The trial will enrol 800 dogs at sites around the US, and last for several years. Enrolment began in 2017, and it will be another two years before the researchers have the first set of efficacy data.
Canine clinical trials are an uncommon first step towards approving human cancer drugs, but it’s a strategy that has worked before. One immunotherapy already crossed the canine–human treatment divide: Mifamurtide (marketed as Mepact by Takeda) for use against bone cancer. It was first shown to work well in dog clinical trials, then tested in humans. The vaccine dose administered to a dog is the same as the dose given to humans, and in most cases veterinary clinical trials in the US are run by the same government agencies that oversee human clinical trials under similar levels of regulatory scrutiny.
The researchers know their goal is ambitious and are readily prepared to accept the trial as a failure. ‘It’s been met with a lot of skepticism,’ admits Thamm. When Johnston and Thamm present their research to other academics and discuss their scientific rationale the outright hostility is usually quelled. ‘They’re now saying “I still don’t think it’s going to work, but there’s enough here that it’s worth trying”.’
The research community might yet to warm to Johnston’s ideas. An October 2019 Nature outlook hailed messenger RNA therapies and cancer vaccines as an ‘exciting field to watch’. It’s not yet clear whether therapeutic or preventative cancer vaccines will be the future, but scientists are invested in both. ‘I think we’ll see a lot of progress in therapeutic vaccines within the next five years,’ Disis predicts.
Claire Jarvis is a science writer based in Atlanta, US







No comments yet