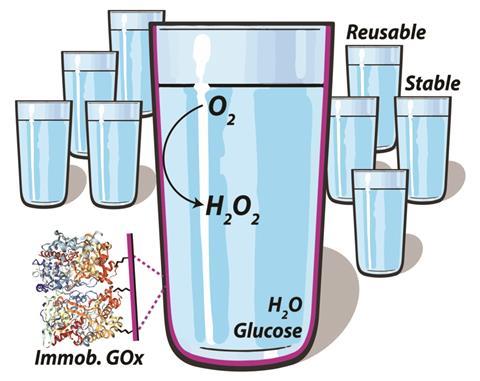
Lab vials coated with glucose oxidase convert dissolved oxygen into a catalyst for RAFT polymerisation
A new range of glassware removes oxygen from solution. The flasks, pipettes and measuring cylinders are activated by adding just a pinch of sugar, and chemists can use them to do air-sensitive polymer-forming reactions without a pump, a Schlenk line or even a lid on their reaction vessel.
All regular solvents contain some dissolved oxygen. Many reactions are sensitive to oxygen, particularly polymerisations, as it can quench the process or generate unwanted side products. Chemists typically remove oxygen by bubbling inert nitrogen or argon through the solution, but they also use vacuum pumps, Schlenk lines and glove boxes. These techniques are tricky, time-consuming and expensive, but with the new glassware, they will be able to do oxygen-sensitive reactions more easily.
Greg Qiao, from the University of Melbourne, Australia, and his team made the glassware by coating the inside surface of standard lab vials with an aldehyde, before adding glucose oxidase solution. The aldehyde quickly reacts with glucose oxidase’s amine groups, conjugating it to the glass surface. It works with any type of glassware.
‘We were working on RAFT polymerisation reactions, which are air sensitive, and one day we thought it would be really cool to make glassware that can actually remove the oxygen itself,’ Qiao says. ‘The glassware works because a redox reaction takes place – the added sugar gets oxidised to an acid, and the oxygen in solution gets reduced to hydrogen peroxide, which forms hydroxyl radicals,’ he continues. RAFT polymerisations involve a reaction between free radicals and a dithioester RAFT agent. The hydroxyl radicals attack the RAFT agent, kicking off the reaction.
‘It’s just like doing laundry. You add the sugar like you add your detergent, and it works to remove dirt from your clothes – or in this case, to remove oxygen from the solution,’ explains Qiao. He says that chemists can use the glassware for any reaction where sugar doesn’t cause a problem, and it could be sold commercially further down the line.
Polymer chemist Tamaki Nakano, from the University of Hokkaido, Japan, describes the process as an amazing invention. ‘It immediately reminds me of Marty McFly’s self-drying jacket and self-lacing shoes from Back to the Future Part II – it saves you the trouble of removing oxygen from your reaction systems forever.’
You can even reuse and recycle the glassware. ‘It shows the power of glucose oxidase yet again,’ says Robert Chapman, an enzyme and polymer scientist at the University of New South Wales, Australia. ‘Not only does it remain active after being immobilised on the glass, it works in up to 50% methanol after more than nine cycles, or after storage at room temperature for 45 days.’ Nakano also says that the reusability is good, but adds that the glassware needs further stability improvements to see widespread use.
Qiao says their next step is to come up with new ways to mix the sugar and enzyme together. ‘We’re working on immobilising the sugar onto the glass as well. Or we could coat both onto the surface of small glass beads and dip them into the solution in a porous bag – just like a tea bag.’
References
This article is free to access until 21 August 2019
M D Nothling et al, Chem. Commun., 2019, DOI: 10.1039/c9cc03477c
















No comments yet