Symmetric compound self-assembles into holey lattice that can self-heal and store gases
Not every complex material needs consist of complex building blocks, as a material made from just a single propeller-shaped molecule has shown. The compound arranges itself into a complex crystal whose pores can even self-heal after they have collapsed at high temperatures.
Most porous materials – like metal–organic frameworks (MOFs) or other coordination polymers – are made from linkers and connecting units. Both are often custom-designed to make materials with pores that can, for example, store hydrogen. But there are thousands of options for each of the two components – so many that scientists have resolved to virtually screen them as it would take years to make and test each of them.
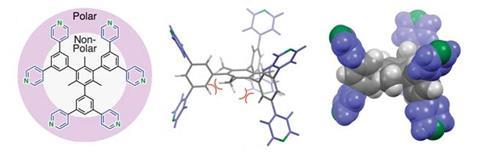
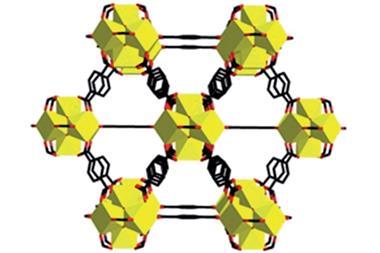
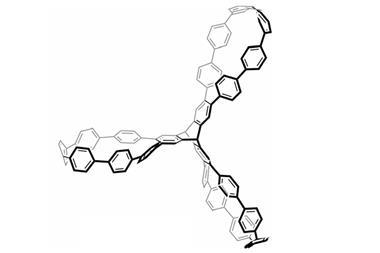
But researchers from Japan have realised that they can make similarly complex structures from just a single compound. Their amphiphilic, propeller-shaped molecule is both linker and connecting unit in a porous network. Each of the propeller arms acts as a different part of the roofs, walls and floors around the 6Å wide pores that can accommodate solvents or nitrogen gas.
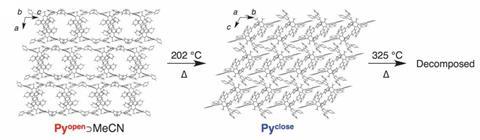
The symmetric compound consists of three polar dipyridylphenyl units connected to a central non-polar mesitylene. In acetonitrile or isopropanol, it self-assembles into a network held together mostly by non-classical hydrogen bonds. Although these C–H⋯N bonds are far weaker than regular hydrogen bonds, the material is stable up to 202°C – most such crystals start to degrade at around 130°C.
Above 202°C, the crystal’s pores collapse and it becomes non-porous. But when cooled down to room temperature and treated with acetonitrile vapour, the material’s pores regenerate.
References
H Yamagishi et al, Science, 2018, 361, 1242 (DOI: 10.1126/science.aat6394)








No comments yet