Singlet cyclopentadienyl zwitterion is the first genuine example of a small antiaromatic molecule
The smallest antiaromatic molecule ever made confirms speculation that previous examples weren’t genuine antiaromatics.1
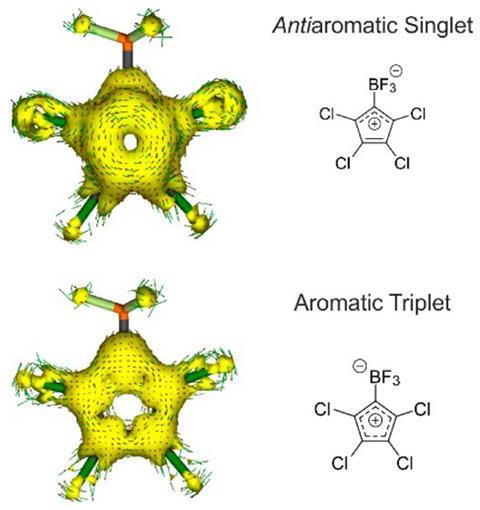
Aromatic molecules with 4n+2 π electrons are more stable than their non-aromatic counterparts. But antiaromatics have only 4n π electrons and are particularly unstable, so much so that the three smallest possible textbook examples – the cyclopropenyl anion, cyclobutadiene and the cyclopentadienyl cation – are all suspected of not being truly antiaromatic.
The cyclopropenyl anion is thought to be non-aromatic,2 and cyclobutadiene’s instability comes from its strained structure rather than from being antiaromatic.3 In 2007, researchers found that the cyclopentadienyl cation’s ground state is a triplet. According to Baird’s rule, only molecules with a singlet ground state are genuine antiaromatics.
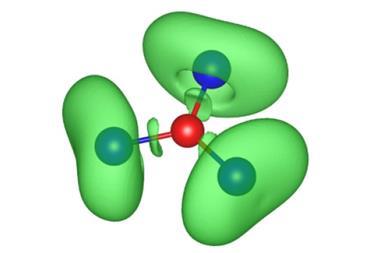
Now, German researchers have made a tetrachlorocyclopentadienyl zwitterion (a neutral molecule carrying separate positive and negative charges) with a singlet ground state. The compound proved to be highly unstable, rearranging into a more stable molecule under the infrared light the team used to study it even at –248°C (25K) in solid argon.
Nevertheless, the team’s spectroscopic measurements show that the zwitterion is a real antiaromatic, making it the smallest of its kind. It might even become the new textbook example illustrating the nature of antiaromaticity.
References
1 P Costa et al, J. Am. Chem. Soc., 2017, DOI: 10.1021/jacs.7b05807
2 S R Kass, J. Org. Chem., 2013, 78, 7370 (DOI: 10.1021/jo401350m)
3 J I-C Wu et al, Chem. Commun., 2012, 48, 8437 (DOI: 10.1039/C2CC33521B)
4 H J Wörner and F Merkt, J. Chem. Phys., 2007, 127, 034303 (DOI: 10.1063/1.2748049)







No comments yet