A reaction that kicks out a single nitrogen, oxygen or sulfur atom from six-membered rings using only blue light has been developed by US scientists.
The method involves breaking the C–N, C–O or C–S bond in saturated heterocycles and reclosing the ring to create smaller cyclic structures. ‘This avoids having to go back to the very beginning of a synthesis to build the type of scaffold that can be accessed here,’ says Richmond Sarpong from the University of California, Berkeley, who led the study.
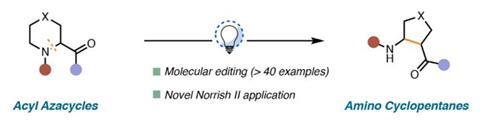
Saturated heterocycles are found in many drugs and agrochemical products; being able to modify them at a late stage opens the way to new chemical space and function. Sarpong points out that ring contractions are often difficult to achieve and usually require high-energy intermediates. Now, his team and collaborators at Merck have come up with a simple strategy that takes advantage of the Norrish type II reaction – a photochemical process involving ketones.
The scientists used blue LEDs to excite an aromatic ketone group attached to the heterocycle, initiating the Norrish reaction that cleaves carbon–heteroatom bond. ‘The second step is a ring closure that is very much a Mannich reaction in the nitrogen case or an aldol reaction in the oxygen case,’ explains Sarpong. ‘These are well-established organic chemistry reactions of carbonyls.’ He adds that finding the right wavelength was key to the discovery. ‘We very much walk a fine line by choosing blue LEDs that emit at exactly the wavelength to excite the carbonyl in the starting material but not in the product.’
‘The authors have developed a unique photochemical reaction that allows you to completely change the shape of a molecule under very mild conditions,’ comments organic chemist Daniele Leonori at the University of Manchester, UK. ‘The process destroys the six-membered ring but cleverly generates adjacent functionalities that react immediately, regenerating a smaller five-membered all-carbon ring.’
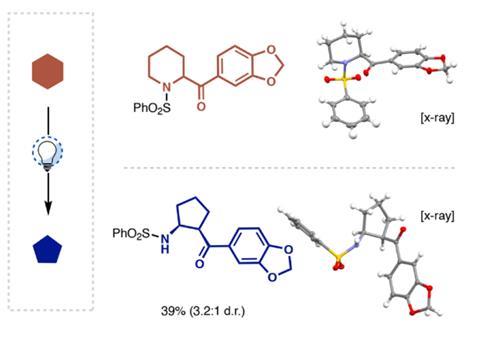
Leonori adds that the methodology ‘offers a brand-new way’ to repurpose building blocks commonly found in medicinal chemistry libraries. Sarpong’s team demonstrated the reaction’s potential for late-stage derivatisation by editing several commercial medicines such as the bronchodilator rimiterol or the antimalarial mefloquine.
‘There are several ring contractions, but the generality here is noteworthy – also the clever differentiation between the reactivity of the two rings in the way they interact with light, which has not been accomplished in this sense before,’ says Tobias Ritter of the Max Planck Institute for Coal Research in Germany.
Sarpong points out that the process is enantioselective but admits that it’s limited by the need for the peripheral aromatic ketone group. But he is convinced that the strategy is an important step towards single-atom swapping – one of the dream reactions on the organic chemists’ wish list. ‘Especially when one considers the ring-opened intermediate where another atom could be inserted in place of the heteroatom that existed in the starting aliphatic heterocycle.’
Sarpong also believes that the reaction could be combined with other approaches such as a recently discovered nitrogen-deletion reaction. ‘This could be a powerful 1–2 punch for core modifications,’ he says.
References
J Jurczyk et al, Science, 2021, DOI: 10.1126/science.abi7183










No comments yet