Ben Valsler
This week, Harriet Brewerton on the household pest killer that may be threatening our friendly pollinators.
Harriet Brewerton
In the 1990’s there were alarming reports of healthy bee colonies failing in France. The ground near beehives was said to be carpeted with dead bees. Similar incidences cropped up around the world, and in 2006 beekeepers in North America reported losses of up to 90%, as forager bees simply failed to return to their hive, causing the colony to collapse.
Global bee populations are declining each year, with some areas regularly losing 50% of their hives over winter.

Aside from producing honey, bees are vital for the world’s food production, and in the UK alone insect pollination is worth around £690 million a year. Declines in global bee populations have prompted the formation of organisations such as the Bee Informed Partnership in the US, who monitor and respond to colony collapse events. Causes for these colony collapses are complex but scientists are starting to unravel the mystery, and one of those causes could be related to a product in your own home right now.
A French company first synthesised fipronil in 1987. It is a unique phenylpyrazole that contains a trifluoromethylsulfinyl group and is a powerful insecticide. It is used in cockroach traps, and is the main active ingredient in many spot-on treatments used by cat and dog owners to control fleas, ticks and ear mites.
Spot-on treatments typically comprise only one tenth fipronil – the rest is oils that spread the insecticide across the skin. As it spreads, the oil-fipronil mix forms wells in the sebaceous glands that exist just below the surface all over the animals’ skin. These glands work to produce a fatty substance to protect the skin, and the oil-fipronil wells get incorporated into this natural process, replenishing the levels of fipronil on the skin surface for days. This allows the very small dose of fipronil in the spot-on solution to not only kill all the insect pests on our pets, but then prevent reinfestation for up to a month.

To achieve this level of cover, fipronil has to be very potent, and this power has made it a promising insecticide for crop protection. It had been deemed safe to use on seeds, to protect sunflowers and other crops from ants and flying insect pests as they grew. It was very effective, but the regulatory tests at the time missed a key fact. Even the tiny amounts of fipronil remaining in the crops could adversely impact pollinator insects and bioaccumulate to reach toxic levels that can affect behaviour, including forager bees’ ability to navigate. Research has linked fipronil use with the 1990’s colony collapses in France, and it has been implicated in other reported bee losses. The accepted LD50 toxicity value of fipronil to bees is 4ng/bee. Ainsley Jones at Fera Science Ltd explains what this means in real-terms: ‘A single dose of 1g would kill 125 million bees, or 4000 bee hives.’
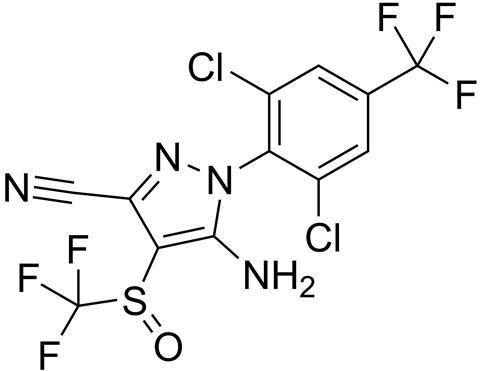
Unfortunately fipronil effects all insects, and does not differentiate between insects considered to be pests by farmers, and insects that are useful pollinators. Fipronil and its oxidation product, fipronil sulfone, work by binding to chloride channels in an insect’s central nervous system, specifically those gated by Gamma-aminobutyric acid (GABA), and glutamate. The opening and closing of these channels controls nerve cell firing which in turn controls the insect’s muscles. With fipronil bound, the gate inhibitor is blocked and the chloride channel remains open. This leads to loss of coordination, paralysis and the eventual death of the insect.
Fipronil binds to insect GABA-gated channels in preference to those in mammals, and glutamate-gated channels do not occur in mammalian central nervous systems. This means fipronil is over 500 times more toxic to insects than mammals, which is why small amounts are safe to use on pets in our homes. The World Health Organisation defines fipronil as only ‘moderately toxic’ to humans, but large doses could cause nausea and dizziness, with long-term effects on the kidneys and liver. Fipronil in the food chain is closely monitored and no such side-effects have been reported from household use.
After decades of research, the main concern with fipronil use remains to be its high toxicity to bees. Colony collapse is a complex issue, and understanding the impact of fipronil, other pesticides and environmental pressures on colonies is helping us to find ways to reverse the trend. In 2013, governing bodies started outlining strict guidance on the data for bees that needs to be gathered before new pesticides are approved for use. Selwyn Wilkins at Fera explains what information scientists now realise is important.
‘The things that were identified were not only the acute contact and oral tests but the need to look at chronic toxicity, so over a longer period for the adult bee. Also laboratory testing to look at in vitro lava rearing, so looking at effects on brood development. And also looking at bumble bees and solitary bees.’

Europe banned the use of fipronil on crops in 2017, with China following suit last year. The potency of fipronil may be difficult to contain for large-scale agricultural use, but on the small-scale it remains a useful compound that I, and thousands of pet owners, use to keep itchy fleas out of our homes.
Ben Valsler
That was Harriet Brewerton with fipronil. Next week, a chemical route to a lustrous full beard, with Catherine Hodges.
Catherine Hodges
With the current beauty trends for thick eyebrows and full beards, more and more people are using minoxidil not just to replace what they’ve lost on top, but to enhance and supplement their facial hair.
Ben Valsler
Join Catherine next week. Until then, find all of our previous podcasts at chemistryworld.com/podcasts, and you can drop us a line with any compounds to cover: email chemistryworld@rsc.org or tweet @chemistryworld. I’m Ben Valsler, thank you for joining me.
Additional information
Theme: Opifex by Isaac Joel, via Soundstripe
Additional music: Witches Brew by Be Still The Earth, via Soundstripe












No comments yet