Meera Senthilingam
This week, the compound that keeps us smelling fresh and fragrant for longer. Sniffing out the chemistry is Josh Howgego:
Josh Howgego
Muscone, as you might have guessed, is the molecule behind the smell of musk. I tend to associate musk with the sort of talc my mum usually buys my grandparents for Christmas, but don't let that put you off. Parfumiers usually describe an authentic musk smell as 'sensuous' and 'intense'. So not normally terms that one would associate with an elderly relative.

However, musky notes do turn up in a whole range of perfumes including Chanel no. 5, so it's not just cheap talcum powder that we should associate with this molecule.
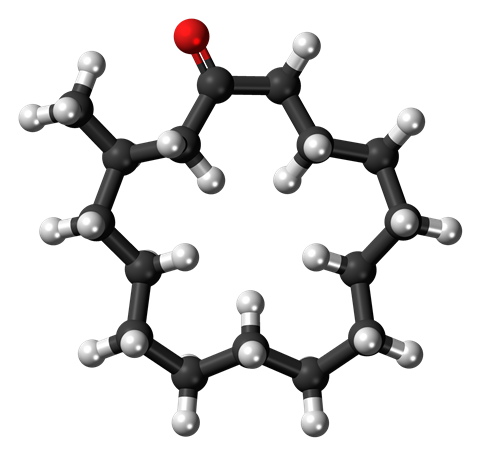
To understand why muscone is such a widely used odourant in perfumes, let's take a look at its chemical structure. This molecule has a fascinating, if not particularly complex structure. It is a ring of 15 carbons containing a carbonyl group with a methyl group at the alpha position - that is, next to the carbonyl. What's interesting about it is the size of that ring. At 15 carbons long, the molecular weight is 224, which, for a smelly molecule, is really very big.
To be smelly, a molecule has to be able to evaporate easily, so that it can waft up and into our noses. The general rule is that the larger a molecule is, the less volatile it is. But if all the perfume evaporated as soon as you sprayed it on, you'd feel a bit short-changed when you arrived at your date, or business meeting, smelling fairly normal. So to get a perfume right, the designers need to perfect the mix between molecules which evaporate quickly (the so-called top notes) and those which linger a little longer (the middle and bottom notes).
Muscone's large size and relatively low volatility make it an excellent bottom note, and it also acts as a fixative, helping to trap some of the other, lighter molecules in the perfume, meaning that you can smell them, too, for longer.
The problem with muscone is where to get it from. Traditionally, it was collected by hunters of Asian musk deer. These animals have glands that secrete the substance and one deer would yield about 25g of dried musk glands which could be used to infuse perfumes.
As an aside, the civet cat also produces a molecule which we call civetone, and this has a very similar chemical structure and smell to muscone. These days, civet cats are fed coffee beans. These pass through the cat without being digested. En route, they are infused with civetone, and are then excreted. After a thorough cleaning - of course - the beans will make a delicious smelling coffee, which connoisseurs buy for ?25 a cup.
But back to muscone. Getting hold of this precious molecule by slaughtering deer is ethically questionable and certainly inefficient. So chemists have tried to do what chemists do best. And that is to find a way to synthesise this natural product quickly and cheaply.
But this is no easy task. As a perfume, muscone's ring size is its most crucial asset, but when it comes to synthesis, it causes all sorts of problems. And the main obstacle is entropy. At some stage in the synthesis, a 15 carbon long chain will have to fold around on itself and react at both ends simultaneously. And persuading such a large molecule to perform this contortion is very hard to do.
Chemists have done it however, starting with another smelly molecule, citronellol. That's the molecule which you can use to keep midges away when you're out camping. Chemists used citronellol to synthesise a 17 carbon chain with double bonds at both ends. Then, using the catalyst that won Robert Grubbs his Nobel prize, the ends come together and react, spitting out the extra two carbons as ethylene gas.
However, this synthesis is time consuming and Grubbs' catalysts are made from expensive ruthenium. So in practice, most contemporary perfumes actually use molecules that smell like muscone, but that don't come from musc deer and, in fact, don't even look like muscone.

In the late 1880s, scientists were interested in making new, more powerful versions of TNT. One such scientist, Albert Baur, synthesised a molecule, which although disappointingly poor as an explosive, just happened to smell quite a lot like muscone. Baur's 'nitro-musks', as they became known, were used extensively in perfumes until the middle of the 2oth century. However, it was eventually realised that they are environmentally persistent, so they're not used anymore.
Today, it's the synthetic compound galaxolide that gets used - which smells like musk, but, again, has a very different structure. Either that, or ambrettolide, which is structurally very similar to civettone, but is extracted from plants.
So in this podcast we've met no less than five molecules, all with completely different structures, but which all smell the same. Elsewhere in the world of molecular interactions, structure and function are intimately linked, but these compounds appear to ignore this rule. So to finish, I'd like to pose a question that's been perplexing scientists for years: If smell doesn't depend on molecular structure - what does it depend on?
Meera Senthilingam
A question that's no doubt got scientists hunting all over the world for the answer. That was Bristol University's Josh Howgego with the fragrance-trapping chemistry of muscone. Now, next week: a compound that's kept it's place in a much-loved Christmas or birthday present.
Brian Clegg
Once upon a time, one of the most exciting gifts you could buy for a child was a chemistry set. It wasn't just the exotic glassware and the promise of producing strange smells, colours, and, if at all possible, explosions. It was the glorious compounds that awaited in little tubes, one of which would almost certainly be potassium permanganate. Today's chemistry sets are ghosts of their former selves. You are more likely to find baking powder and citric acid than serious chemical supplies. But you can still find sets packing those beautiful purple crystals with their near-metallic sheen.
Meera Senthilingam
And to find out just what these purple crystals are used for, by both amatuer and professinal scientists, join Brian Clegg in next week's Chemistry in it's element. Until then, thank you for listening. I'm Meera Senthilingam.













No comments yet