Kat Arney
PARP, or poly-ADP-ribose polymerase to give it its full name, is a protein found inside our cells acting as a vital early warning system for damage to DNA. Ignoring the slightly silly name, not only is it a fundamental part of the way in which our bodies protect themselves against cancer, it’s also the key to powerful new treatments for the disease.
PARP lives inside the cell’s nucleus alongside DNA. It patrols the DNA, looking out for certain types of damage known as single-strand breaks. If you imagine the DNA double helix as a twisted ladder, these breaks would be in just one of the struts. As soon as a single-strand break is spotted, PARP attaches to the broken DNA and gets to work, creating long chains of a sugary compound called poly-ADP-ribose. This acts as a red flag to the cell’s DNA repair systems, calling them in to fix the damage. It’s a powerful protection mechanism, helping to spot and fix potentially cancer-causing mutations in DNA before they become a problem. But it’s not perfect. In fact, more than one in three of us are likely to get cancer at some point in our lifetime.
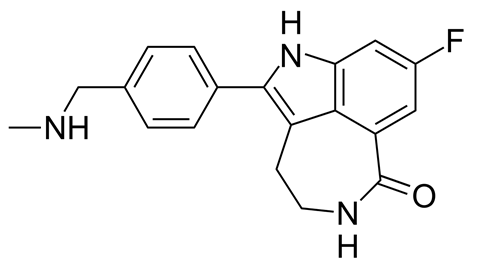
However, scientists have now worked out how to harness the power of PARP as a weapon against the disease, by switching it off. This relies on two more, important proteins and the genes that encode them – BRCA1 and its molecular cousin, BRCA2. When they’re functioning normally, BRCA1 and BRCA2 are also involved in DNA repair. People with faulty versions of either BRCA gene are at a greatly increased risk of breast, ovarian and prostate tumours, because they can’t repair DNA damage properly in these tissues, leading to a build-up of cancer-causing mutations. These cancers often tragically appear in younger people, and run in families due to the inheritance of the faulty gene.
But although the BRCA repair systems may not be working properly in tumour cells, other repair systems – including the one run by PARP – are picking up the slack. This gave scientists a good idea for a completely new way of attacking cancer, known as synthetic lethality. In 2005, researchers Alan Ashworth and Steve Jackson discovered that substances that stop PARP from working (known as PARP inhibitors) could kill cancer cells in their tracks, if they also carried a fault in BRCA1 or BRCA2. Without their back-up PARP system, the cancer cells have no way of repairing damaged DNA, so they die. This combination of failures – one genetic, the other chemical – is lethal.
Over the intervening decade, researchers worked hard to develop these PARP-blocking compounds into suitable drugs for patients, and in 2015, the first PARP inhibitor, olaparib – also known by the brand name Lynparza – was approved for use on the NHS in the UK for women with certain types of ovarian cancer due to BRCA gene faults. Several other drugs are following hot on its heels, although clinical trials have had mixed results. One drug – iniparib – failed badly in trials, leading to questions about whether PARP inhibitors really work for treating cancer, but on closer examination iniparib turned out not to actually block PARP at all. Oops.
There may be benefits for PARP inhibitors outside the relatively small group of patients with BRCA gene faults. Over the past few years, the cancer research community has been abuzz with talk of ‘BRCA-ness’ – cancers having properties similar to those with BRCA faults, perhaps having faults in other DNA repair genes that are also sensitive to PARP inhibitors. Olaparib and other drugs are being tested for several other types of cancer, including lung, prostate, oesophageal and pancreatic cancers, as well as brain tumours and blood cancers.
One thing to notice about the story of PARP inhibitors is that it was remarkably fast, taking little more than a decade to go from a twinkle in a scientist’s eye to a drug on the shelves. That may seem like a long time, but it’s fast compared with some pharmaceutical journeys. Let’s hope that this story of working smarter and faster sets the scene for the cancer drugs of the future.
Ben Valsler
Kat Arney on the rapid development of anti-cancer drugs based around inhibition of poly-ADP-ribose polymerase. Next week, Louise Crane explains why certain American chocolates don’t appeal to European palates…
Louise Crane
American chocolate fans are so used to the sour, cheesy taste that other chocolate manufacturers who don’t use the Hershey process actually add butyric acid to give their chocolate that distinctive, long-lasting tang - which lovers of sweet, creamy British chocolate describe as tasting like parmesan cheese or even baby sick.
Ben Valsler
Join Louise next week to find out why Hersheys has that distinctive flavour. Until then, let us know if there are any compounds we should cover by emailing chemistryworld@rsc.org, sending us a message on facebook or tweeting @ChemistryWorld. I’m Ben Valsler, thanks for joining me.












No comments yet