First non-classical hydrogen bond involving boron–hydrogen to π-system observed
An entirely new class of hydrogen bond that forms between a boron–hydrogen group and the aromatic, p-electron system of a benzene ring has been discovered. The non-classical B–H…p bond can be seen in the gas phase locking together diborane and benzene with a strength comparable to the hydrogen bonds that hold water dimers together.
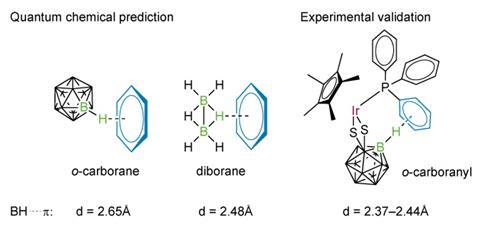
Dieter Cremer and Wenli Zou of the computational and theoretical chemistry group at Southern Methodist University, Dallas in the US, worked with coordination chemists from Nanjing University in China to investigate the theory of non-classical hydrogen bonds that might form between a B–H group and organic structures and to demonstrate one such system experimentally.
Non-covalent bonds between aromatic rings and a hydrogen joined to either a carbon, nitrogen or oxygen atom, are common and critical in molecular biology. These relatively weak electrostatic bonds are the currency of countless biomolecular interactions between proteins, nucleic acids and the milieu of molecules on which life relies. Theoreticians and experimentalists have looked closely at many of these bonds and modelled the likes of benzene–haloalkane, benzene–ammonia and benzene–water complexes. Such bonds in, for example, carborane systems have hinted at a new approach to drug design in which a B–H group interacts with a proton donor in a target biomolecule. However, an interaction between a B–H and an aromatic ring had not been observed until now.
The team points out that given the usual trend with such systems one might expect boron’s electropositive character to make any interaction between a B–H compound and an aromatic ring repulsive rather than attractive. Of course, the bonding in diborane is itself rather unusual with its two-electron, but three-centre motif. The team suspected that the B–H group that might otherwise be repelled by a benzene ring would, in the case of diborane, be stabilised because the bonding hydrogen atom would have a residual positive charge.
The team’s quantum chemical calculations suggest that just such a bond should exist and that the interaction would be electrostatic. They generated the non-classical bond between diborane and an aryl group in a phosphine derivative of iridium-dimercapto-carborane complex. X-ray diffraction revealed the distance between the hydrogen atom and the p ring system to be between 2.40 and 2.76Å.
Cremer tells Chemistry World that the iridium complexes seem to form oligomers, so the next step in their work will be to look for the possibility of making compartments. ‘We are also looking at the possibilities for switching the B–H H-bond on and off,’ he says.
‘Chemists are still fascinated by hydrogen bonding interactions almost 100 years after Latimer and Rodebush first proposed the now classic concept,’ says Scott Cockroft of the University of Edinburgh, UK, who has investigated the strength of non-covalent interactions. ‘This work by Yan, Cremer and co-workers adds another example to the growing menagerie of non-classical hydrogen bonds that involve atypical H-bond donors and acceptors.’
References
X Zhang et al, J. Am. Chem. Soc., 2016, DOI: 10.1021/jacs.6b01249








No comments yet