Vaccine raises hopes of an end to Ebola
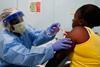
Trial shows vaccine provides complete protection and points way to fast-tracking medical research

Trial shows vaccine provides complete protection and points way to fast-tracking medical research