A systematic survey of archived protein structures has revealed dozens of examples of a previously overlooked covalent bonding motif. Mapping these nitrogen–oxygen–sulfur (NOS) linkages could open the door to new therapies and better engineering of proteins.
Despite huge protein databanks and use of AI tools such as AlphaFold trained on these data, the findings suggest that important interactions between amino acids have been missed.
In the new research, x-ray crystallographic data from 86,000 protein structures was analysed with machine learning and quantum-mechanical calculations to uncover 69 previously unreported NOS bonds.1 ‘I’m surprised that this gap was missed for so long – 50 years of experimental determination of protein structures,’ says Sophia Bazzi, a researcher at Georg-August University Göttingen in Germany, who led the project.
An NOS bridge between a cysteine and a lysine amino acid had been first reported in 2021 by a group at Göttingen interested in targeting the bacteria that causes gonorrhoea.2 This link acted as a loaded-spring mechanism that changed the shape of the protein depending on its redox state. A survey of the protein data bank then turned up more NOS bridges across many protein families.
The new tool, developed by Bazzi and her colleague Sharareh Sayyad, searched structural data from the Protein Data Bank for anomalies that could signify the presence of overlooked NOS bonds. Specifically, it looked for residue pairs featuring sulfur and nitrogen atoms positioned close to each other.
The endeavour revealed NOS links between lysine and cysteine; arginine and cysteine; and glycine and cysteine. These had been overlooked or misclassified, for example as ‘too-close contacts’ marking their proximity as suspicious.
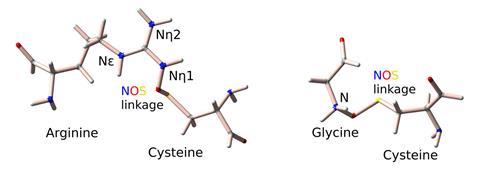
Much of a protein’s shape is determined by non-covalent forces such as hydrogen bonds, electrostatic interactions and van der Waals forces. Disulfide bonds between two cysteine residues are, however, an example of covalent bonds in proteins that influence structure. These can be reversibly cleaved, allowing them to act as redox switches that change a protein’s shape and activity.
Adding NOS bonds as a category of redox switches could open up new therapeutic targets. In early 2024, a collaboration including Bazzi reported that a cysteine protease in Sars-CoV-2 contained redox switches that allowed the enzyme to be structurally stable under oxidising conditions, protecting them against an immune system response to viral infections3. The team proposed that interfering with the switch could inhibit the viral enzyme.
However, not everyone is convinced that NOS bonds are dynamic. ‘This is a strong study and these motifs clearly exist,’ says Philip Hogg, a protein chemist at the Centenary Institute in Sydney, Australia. ‘They could be structural bonds that help define protein shape, but there isn’t evidence as yet that they can be broken and reformed to act as switches.’
‘If it transpires that there is an enzyme or factor that could do that, that’ll be a big discovery,’ Hogg adds. ‘In terms of their therapeutic potential, it really does hinge on them being dynamic functional switches. It would not surprise me if they were dynamic, but current evidence is that they’re static bonds.’
Bazzi notes that when crystallographers translate electron density maps into protein models, they tap chemical libraries and predefined rules on interactions. These 3D protein structures are then used to train AI models that are applied in further research.
‘We rely too much on the protein data banks and when the [electron density] maps say there is something wrong here, something is missing, people don’t pay enough attention to that,’ she says. ‘But if a bond is missing from the data, no matter how intelligent the AI is, it can never predict it.’
Bazzi is now moving beyond NOS to search published protein structures with the goal of uncovering more hidden protein chemistry, including overlooked chemical interactions and covalent modifications.
References
1. S Bazzi and S Sayyad, Commun. Chem., 2025, DOI: 10.1038/s42004-025-01535-w
2. M Wensien et al, Nature, 2021, DOI: 10.1038/s41586-021-03513-3
3. L-M Funk et al, Nat. Commun., 2024, DOI: 10.1038/s41467-023-44621-0













No comments yet