
A novel cocktail of synthetic proteins, inspired by the unique antibodies of alpacas and llamas, can neutralise toxins from some of Africa’s most dangerous snakes. The work raises hopes for a universal, low-cost antivenom that could protect against the hundreds of venomous species of snakes.
Snakes are responsible for killing over 100,000 people each year worldwide, with many more victims suffering permanent disabilities. Their venoms are a complex mixture of many compounds targeting nerve cells, blood clotting or tissues. ‘Snakes have different venoms and within one venom you have something like 80 different toxins,’ explains toxinologist Andreas Laustsen-Kiel of the Technical University of Denmark, who led the work. Snakebite is now classified by the World Health Organization as a neglected tropical disease, with the greatest burden falling on rural communities in sub-Saharan Africa and south Asia.
Antivenoms can save lives if administered promptly, yet those in need rarely receive them. Snakebites mainly occur in rural areas, where clinics tend to be small, under-resourced and often ill-equipped. ‘Clinicians may underdose because they know antivenom is expensive and a scarce resource,’ explains Nicholas Casewell, at the Liverpool School of Tropical Medicine (LSTM), UK, who contributed to the work.
Composed of antibodies raised against snake venoms, antivenoms neutralise toxins by binding to them or by aggregating them to prevent harm. Traditional antivenoms are made by immunising horses or sheep with non-lethal doses of the venom, then harvesting their antibodies. This time-consuming and costly process produces a heterogeneous mixture of antibodies of varying quality, meaning multiple vials of the antivenom are needed to treat a single patient. ‘If you need to make double the amount of antivenom, currently you need double the number of horses and sheep – the manufacturing process doesn’t scale so well,’ explains Laustsen-Kiel.
Camelid chains
To tackle these limitations, the team turned to alpacas and llamas whose antibodies have a single protein chain instead of the two-chain arms of the typical Y-shaped antibodies found in humans and horses. These nanobodies retain the strong binding power of conventional antibodies, neutralising toxins in the same way, but lack the bulky regions that trigger severe immune reactions in humans, reducing the risk of anaphylaxis seen in conventional antivenoms.
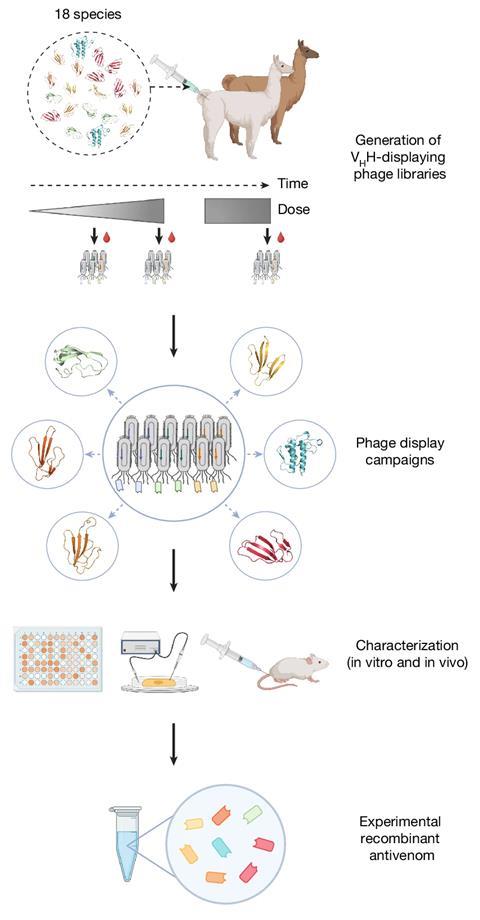
The researchers immunised an alpaca and a llama with venoms from 18 of Africa’s most dangerous snakes, including cobras, mambas and rinkhals. To mass produce the nanobodies, the researchers isolated the DNA sequences that coded for the resulting nanobodies and inserted them into bacteria, turning them into nanobody bioreactors. Eight nanobodies were then selected from thousands by testing them against a wide variety of snake toxins. These formed a potent mix that protected mice from lethal doses of venom from 17 of the 18 species tested. ‘The broad coverage (in terms of species) is a major step forward,’ comments Anita Malhotra, an evolutionary biologist from Bangor University, UK, who was not involved in the work.
This simple synthesis allowed the researchers to fine tune the relative abundance of each nanobody to target various species’ venoms. ‘We have already developed a mix against Latin American coral snakes, and are testing nanobodies we discovered against Indian snakes and African vipers,’ says Laustsen-Kiel. ‘If we change a few components and optimise the combination, we could have a catalogue of nanobodies to hopefully cover 90% of the medically important toxins.’
Using bacterial, fungal or yeast systems as bioreactors means that nanobodies can also be manufactured more cheaply. ‘Looking at the manufacturing cost, we estimate it to be below $100 (£77) per treatment, whereas a full treatment for a snake bite with the existing animal-derived antivenom in sub-Saharan Africa has a sales price close to $1000,’ notes Laustsen-Kiel. ‘The challenge is that this is a completely new type of product, so it has to go through a lot more approval.’
Malhotra notes the need for clinical trials too. ‘It is well known that mice aren’t particularly good models for the effects of venom on humans,’ she says. The small size of nanobodies also means they are cleared quickly from the body, which warrants the need for trials in larger animals. ‘The next step in our work is to look at this in sheep,’ adds Laustsen-Kiel. ‘We need to study the right balance between using techniques to extend the half-life and the resulting complexity and cost of the treatment.’
Conducting clinical trials and setting up biologics production for an antivenom in remote regions with limited infrastructure is also a logistical challenge. ‘The financial business model is a real challenge for products that are potentially going to be specific to a low-resource settings,’ adds Casewell. Still, discussions with pharmaceutical companies, universities and funders, including several serum institutes and the LSTM, are underway. ‘If we can out-license or spin out a company to do this, we’d be very interested,’ Laustsen-Kiel says. ‘Not because we are interested in making money, but because there is an enormous unmet medical need.’
Toxinologist Bryan Fry at the University of Queensland, Australia, who was not involved in the study, describes the work as ‘an absolute game-changer for the antivenom field’. He notes that antivenoms have historically been the classic poverty trap, needed most by the people who can afford them least. ‘If they can come up with a broad enough product, not just pan-African but pan-African/Asian, then the economy of scale could make [translation] realistic,’ he adds.
The number of serum institutes involved in this work was updated on 11 November 2025.
References
S Ahmadi et al, Nature, 2025, DOI: 10.1038/s41586-025-09661-0















No comments yet