A versatile method for making anilines – important aromatic molecules used in drugs and agrochemicals – has been developed by researchers in the UK.1 The new approach, which uses non-aromatic precursors and light, bypasses some of the frequent selectivity issues of aromatic chemistry and enabled the scientists to synthesise compounds that can’t be made using traditional cross-coupling reactions.
Anilines are benzene rings with a nitrogen atom attached. These compounds are widely used in industry and are normally prepared by metal-catalysed reactions involving pre-functionalised aromatic substrates. ‘The Buchwald–Hartwig cross-coupling – one of the best reactions ever – is one of these kinds of transformations,’ says Daniele Leonori of the University of Manchester, UK. Leonori explains that although these cross-coupling reactions are very reliable, obtaining the aryl halide precursors can sometimes be challenging. ‘That’s because the chemistry that we have to insert halogens on aromatic rings has a lot of selectivity rules, so there are many compounds that we can’t make, simply because we don’t have a good reaction.’
To overcome this limitation, Leonori and his team, in a collaboration with the pharmaceutical company AstraZeneca, replaced the aromatic starting materials with single-bonded rings containing a C=O group, called cyclohexanones. ‘Our idea was that we can use carbonyl chemistry, which is very selective, to introduce different substituents around this group,’ he says. The researchers mixed the cyclic ketone with an amine and used two metal catalysts and a blue light-emitting diode to produce the desired aniline. They demonstrated the utility of their new strategy by making several commercial drugs.
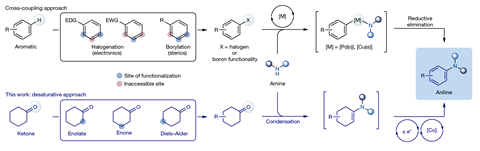
‘The synthetic procedure is really clever,’ comments Warren Cross, a researcher at Nottingham Trent University, UK. ‘The researchers attach a nitrogen atom to a saturated carbon ring and then remove hydrogen atoms from the saturated carbon ring to generate the benzene ring of the aniline. All of this happens in one reaction,’ Cross notes that many substitution patterns can be accessed in this way. ‘This means that a greater range of aniline molecules can be made efficiently and easily using this single method. The huge scope of aniline compounds described in the study is very impressive.’
Shannon Stahl of the University of Wisconsin–Madison in the US, who developed a previous strategy to make aromatic rings from cyclohexanones,2 says that the new approach is ‘impressive in scope and compatibility with mild reaction conditions.’ He explains that earlier methods leveraged the use of oxygen as an oxidant and employed transition-metal catalysts to promote the dehydrogenation of the ring. ‘The present report promotes the dehydrogenation process by using light and a photoredox catalyst to generate a reactive radical, in combination with a cobalt catalyst that evolves hydrogen gas as the byproduct.’
Leonori points out that the new procedure is meant to fill the gap where common cross-coupling methods don’t work. ‘It’s impossible to be better than the current state of the art because those reactions have been optimised over 30 years,’ he says. ‘Our idea was to look at particularly challenging motifs and try to find a helpful strategy.’ John Hartwig at the University of California, Berkeley in the US agrees that the approach could be valuable in such situations. ‘There will be cases where this would be a useful complementary route to making anilines when the functional group array doesn’t allow cross-coupling,’ he says.
Scaling up the synthesis would still require some work. Leonori says that the main problem at the moment is the long time required for the reaction, so the team is trying to find out which step is slowing down the process to improve it. Stahl believes that the iridium photocatalyst loading might have to be reduced too. ‘But this strategy is very compelling,’ he says. ‘I can certainly envision this approach finding use in medicinal chemistry, and there is no reason to believe that further improvement couldn’t be realised via process-development efforts.’
References
1 S U Dighe et al, Nature, 2020, 584, 75 (DOI: 10.1038/s41586-020-2539-7)
2 A V Losub and S S Stahl, ACS Catal., 2016, 6, 8201 (DOI: 10.1021/acscatal.6b02406)











No comments yet