Helical copper compound’s enantioselectivity can be inverted while reaction is running
The first catalyst whose enantioselectivity can be switched back and forth during a reaction could be used to produce bespoke chiral polymers, or create consecutive stereocentres in complex natural products in one pot.
Enantioselective catalysts produce only one of the two possible mirror-image structures in handed molecules. In order to do this, the catalyst itself needs to be chiral – in metal catalysts, this is usually done by attaching chiral ligands to the metal. The ligand’s chirality, however, is fixed and cannot be changed during a reaction.
A dynamic, enantio-switching catalyst has now been created by a team led by Matthieu Raynal from Sorbonne Universités, France. Within only five seconds, their supramolecular copper compound can invert its enantioselectivity for forming chiral alcohols from non-chiral ketones.
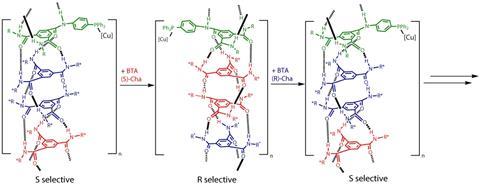
The catalyst consists of chiral benzene 1,3,5-tricarboxamide (BTA) units. In solution, these building blocks form supramolecular helices stabilised by hydrogen bonds; the chiral groups dictate if the helix is left or right handed. Copper, attached to the side of the helix, is the catalytic centre that transfers the helix’s chirality onto other molecules.
‘Our catalyst has chirality amplification properties,’ explains Raynal, meaning it takes on the chirality of its major constituent. Switching the helix from left to right handed, and therefore switching the catalyst’s enantioselectivity, simply involves adding more of the minor BTA enantiomer. To switch it back the researchers add more of the other BTA enantiomer.
To show that their catalyst can be switched in real time, Raynal’s team set up a reaction with two ketones, one that reacts fast and the other slowly. They started off with the catalyst in its S form and switched it by adding more R-BTA after two minutes.
Indeed, the fast-reacting substrate formed 87% of one enantiomeric alcohol, while the slower ketone, which mostly reacted after the catalyst had been switched, formed 67% of the opposite enantiomer. ‘[This] is the most interesting experiment in my view,’ comments Joost Reek from the University of Amsterdam, Netherlands, who works on supramolecular catalysis. ‘It represents a new level of control of catalytic conversion in time and space.’
‘It’s fascinating work, a real breakthrough in the area,’ agrees organometallic chemist Jeanne Crassous from University of Rennes 1, France. Such systems, she says, could allow chemists to tailor-make polymers by repeatedly switching the catalyst during polymerisation.
‘These are very simple examples, but you could imagine having multiple substrates and multiple catalysts in solution that you could switch on and off,’ says Reek. Before this happens, however, switchable catalysts will need to prove that they work in more than one type of reaction. ‘Will this work with other examples? Can this be applied in a more general fashion? These are questions we need to address,’ Crassous concludes.
References
J M Zimbron et al, Angew. Chem. Int. Ed., 2017, DOI: 10.1002/anie.201706757














No comments yet