‘Artificial atoms’ that form molecular orbitals reminiscent of those in well-known organic molecules have been produced by researchers in the Netherlands using strategically positioned caesium atoms on the surface of a semiconductor. The artificial atoms should allow researchers to measure the energies of molecular systems that would be unstable in real life, and thereby potentially gain greater insights into chemical energetics.
The scanning tunneling microscope’s ability to manipulate individual atoms was famously demonstrated in 1989 when Don Eigler and Erhard Schweizer at IBM Almaden in California spelt out ‘IBM’ using 35 xenon atoms on a nickel substrate. In 1993, Eigler and two colleagues then looked at quantised electronic resonances from iron atoms arranged in a circle on copper, and others have subsequently created more complex 2D arrangements akin to atoms and molecules. However, ubiquitous metal substrates have proved a problem. ‘You do create something like an artificial molecule on the surface,’ explains Daniel Wegner of Radboud University in Nijmegen, ‘but it is still strongly coupled to the substrate, and that basically kills a lot of the properties that are intrinsic to what you actually build.’ Removing the conductive substrate, however, is tricky because scanning tunnelling microscopy works by measuring the variation in the quantum tunnelling of electrons between the tip and the surface under an applied bias voltage. If the substrate itself does not conduct electrons, the tunnelling current becomes zero, which makes the tip unable to detect the substrate and thus renders scanning tunnelling microscopy impossible.
In the new work, Wegner and Radboud University colleagues led by Alexander Khajetoorians used the narrow-bandgap semiconductor indium antimonide as a substrate. This has just enough conductivity to allow them to detect a background tunnelling current provided the voltage was high enough. They then added highly electropositive caesium, which released electrons into a bound state in the semiconductor bandgap, forming a ‘2D electron gas’ that did not couple to the bulk material. The researchers arranged the remaining caesium cations into what Wegner describes as ‘an atom without a nucleus’ – a ring that had an electromagnetic potential analogous to that between atoms. ‘There seem to be s- and p-orbitals like for a real atom,’ Wegner says.
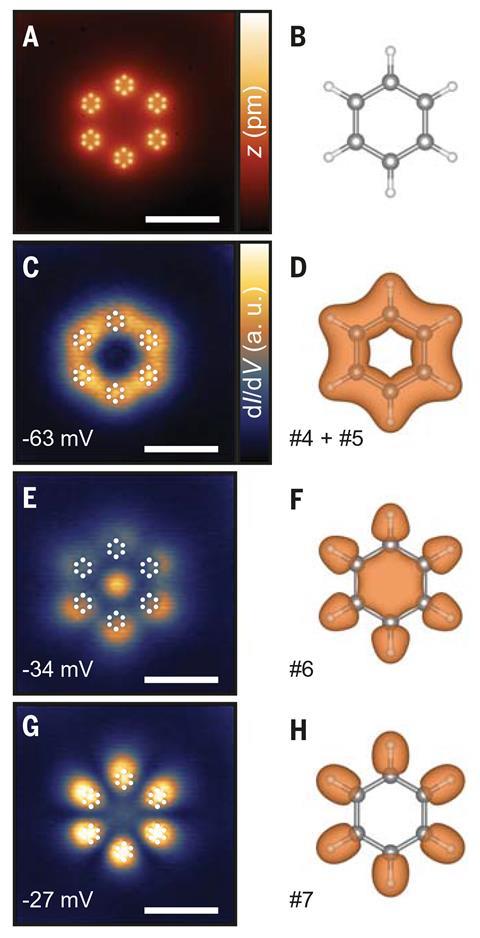
The group went on to arrange these ‘artificial atoms’ into artificial molecules such as cis and trans butadienes and even artificial benzene – finding energetic evidence of sp2 hybridisation even in their 2D model, which Wegner explains is due to the projection of the pz orbitals into the xy-plane.
The researchers now want to use this to explore the fundamentals of chemical bonding. ‘We can now play mind games that previously only a theoretician could with DFT code and just say “what if?”,’ says Wegner. ‘What if I change the bond order of a molecule? A chemist cannot do that: a chemist can only synthesise what nature determines is the ground state. But there is no relaxation of artificial atomic sites – they cannot stabilise and move by themselves: we move them.’ Wegner says that the researchers would ultimately like to discover emergent effects in their system that cannot be explained by the Schrödinger equation.
Saw Wai Hla of Ohio University in the US says the key to the article is the creation of the artificial atom. ‘Once you have that you can create as many as you wish at different distances… It has an atomic potential, so you can temporarily forget about the whole thing: you walk like a duck and you quack like a duck. It’s not the whole story of course – it’s just the beginning, but if you bring them together, they will form a bond, and they do.’ He notes that some complexities of real molecules such as steric repulsion are not captured in a 2D model but concludes that, as an experimentalist, he ‘really likes’ the paper. ‘You can always say “a computer predicts” … this is real!’
References
E Sierda et al,Science, 2023, 380, 1048 (DOI: 10.1126/science.adf2685)














No comments yet