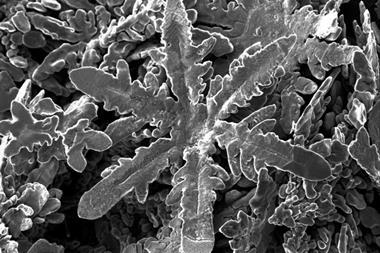
Drops of liquid metal can be grown into snowflake shapes, researchers have discovered.
When a team of scientists from the North Carolina State University, US, tried to use a droplet of liquid gallium–indium as an electrode in a electrochemical cell, they observed something odd: within a few seconds, the metal starts to spread out into a snowflake-like shape. An unusual behaviour for a liquid that has the largest surface tension of any fluid and is therefore rarely inclined to change its shape.
Applying a moderate voltage, however, kicks off a reaction between the gallium alloy and the surrounding electrolyte, a solution of sodium hydroxide in water. The metal’s surface becomes oxidised, which lowers its surface tension in the same way soap lowers the surface tension of water. As gravity spreads the metal out into a disc, disturbances on its surface break it up into individual ‘petals’. As they grow, the petals break up into more branches, creating a fractal pattern.
The largest liquid metal snowflakes are around 20 times the size of the original droplet and form in less than half a minute. However, the researchers found that the effect only works in a narrow voltage window around 2V. At lower voltages, the droplet’s surface doesn’t oxidise enough to disturb its shape. If the voltages are too high, the surface oxide layer grows too thick and doesn’t allow the droplet to spread and break up.
Liquid metals have been used as wheels for miniaturised vehicles and as tiny actuators. Being able to precisely control their shape could help design new types of soft electronics.
References
C B Eaker et al, Phys. Rev. Lett., 2017, 119, 174502 (DOI: 10.1103/PhysRevLett.119.174502)














No comments yet