HPLC-free synthesis slashes protein production time
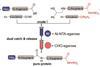
Tagging system may make building biologically relevant proteins faster, cheaper and greener

Tagging system may make building biologically relevant proteins faster, cheaper and greener