Single-atom swaps are rare because strong bonds in molecular frameworks are difficult to break selectively. But Bill Morandi’s group at ETH Zürich in Switzerland has reported a carbon-to-nitrogen swap in N-alkyl indoles, directly converting them into the corresponding benzimidazoles. This one-pot reaction uses simple, commercially available reagents with no need for metal catalysts or protecting groups.
Morandi’s group is a pioneer of skeletal-editing, remodelling molecules on an atomic level to access new areas of chemical space. The latest work demonstrates that complex, drug-like molecules can be transformed in a single synthetic step, something that would be valuable to the pharmaceutical and agrichemical industry.
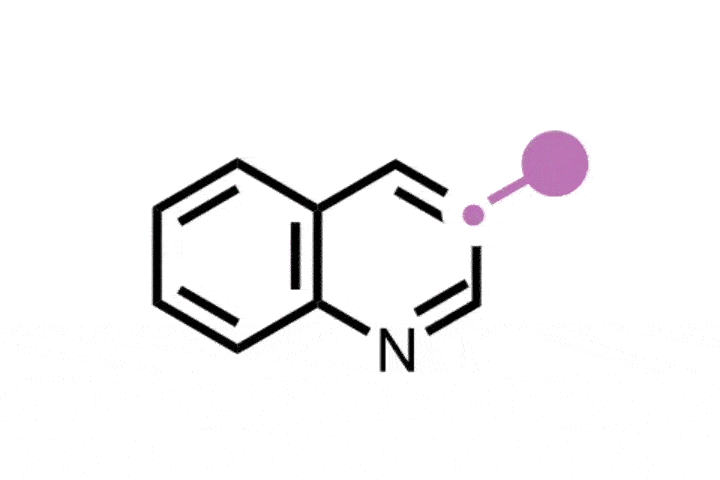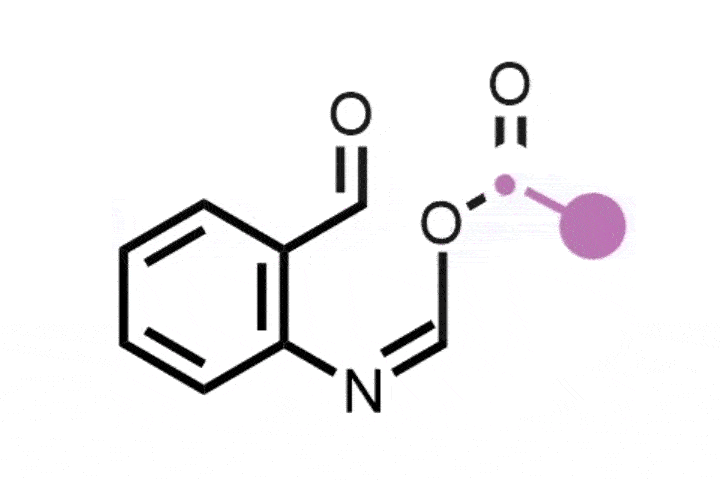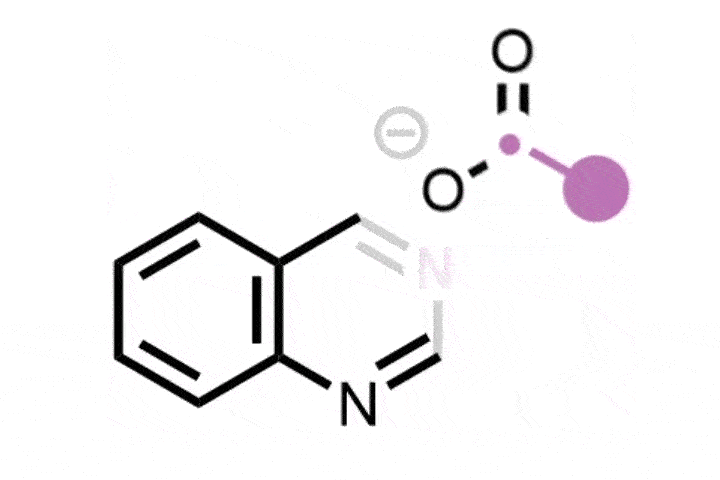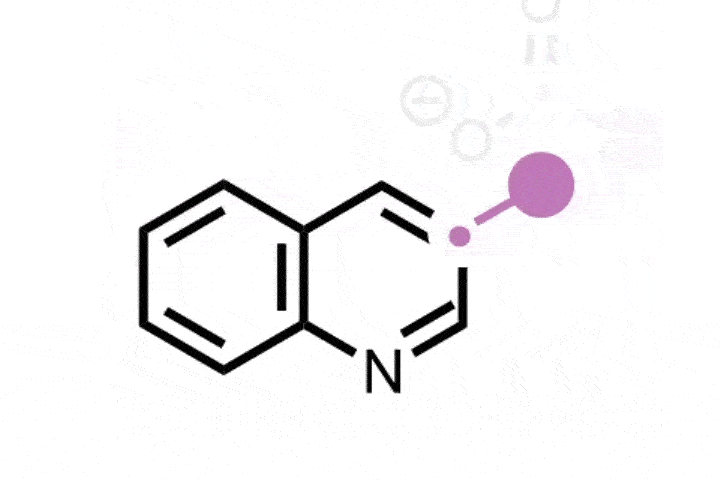
The reaction itself is elegant in its design. It proceeds via a carefully orchestrated sequence: oxidative cleavage to the Witkop intermediate, followed by amide formation and Hofmann-type rearrangement. The sequence is mediated by commercially available reagents, including ammonium carbamate, which serves as the nitrogen source, and phenyliodine(III) diacetate (PIDA). Specificity stems from the selective formation and reactivity of the Witkop intermediate, which guides the transformation with minimum side reactions.
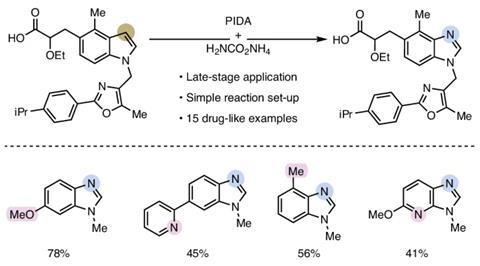
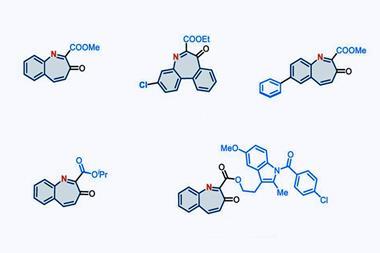
‘The operationally simple reaction setup gives access to complex benzimidazoles within 30 minutes,’ explains Ann-Sophie Paschke, a member of the team. By replacing a single atom in the indole scaffold, a common motif that appears in bioactive molecules, the physiochemical properties of the molecule can be altered and new target binding sites could be introduced. ‘With our approach, native indoles from commercial suppliers or existing libraries can be directly subjected to the reaction conditions,’ adds Paschke.
Unlike traditional approaches for molecular diversification, which often rely on peripheral functionalisation, this approach modifies the core skeleton. No additional functionalisation of the parent indole is required, enabling the use of existing compound libraries. However, single atom swaps typically require the installation of specific functional groups, such as azides or N-oxides, limiting the applicability in late-stage diversification.
The group highlighted this late-stage versatility by applying the reaction to 15 drug-like indoles, ‘highlighting its potential as a useful addition to the medicinal chemistry toolbox’ of molecular editing. Among them, a 5-HT1C antagonist analogue was transformed into benzimidazole, demonstrating the reaction’s compatibility with sensitive functional groups such as urea. A phosphatidylinositol-3 kinase inhibitor also underwent the swap to its benzimidazole derivative with minimal disruption to the sulfonamide’s functionality. These examples highlight the method’s versatility and relevance to real-world drug scaffolds.
Other groups have reported promising strategies for single-atom exchange reactions that expand the toolbox of late-stage editing methodologies. For instance, in morphine, replacing an oxygen in the core E-ring with carbon produced an analogue that retained painkilling activity while reducing side effects, illustrating how precise atomic edits can tune biological function.
Alec Christian, associate principal scientist at Merck, believes this new methodology is ‘unique and is adding to the toolbox’, and says that, ‘one of the most impactful aspects of this work is its ability to adjust the electronics of molecules, which can directly influence their biological activity’. He adds that, ‘it’s rare to have a method that allows a controlled nitrogen insertion at a late stage, directly on complex scaffolds’.
Armido Studer, an organic chemist at the University of Münster, comments that ‘the beauty of this method lies in combining well-known reactions in a single step, directly converting indoles into benzimidazoles without pre-functionalisation’. ‘Indoles are a common scaffold in biologically active molecules,’ he adds, ‘modifying them through single-atom swaps can rapidly generate analogues for drug discovery’. Studer also notes that the technique could ‘expand structural libraries, explore new analogues and even inspire applications beyond pharmaceuticals, such as in materials science’. Work on related C-to-N atom swaps in indoles and benzofurans has also been explored by Studer’s group using a two-step process, offering a flexible route to new heterocyclic scaffolds.2
Looking ahead, Paschke says, ‘we aim to develop further methods to access various heterocycles to facilitate rapid synthesis of novel or hard-to-access scaffolds and to ultimately support the discovery of new bioactive compounds’.
References
1 A-S K Paschke et al, Nature, 2025, DOI: 10.1038/s41557-025-01904-x
2 A Studer et al, Nature, 2025, DOI: 10.1038/s41586-025-09019-6












No comments yet