A new technique for manipulating molecular rotations using the electric fields of light waves could provide insights into electrons’ movements during a chemical reaction.1
Chemists understand in general that reactions occur when electrons lower their total potential energy by inducing atoms to reconfigure. Tracking electrons’ picometre-scale movements over the femtoseconds during which a specific reaction occurs, and understanding what influences the probability of the reaction occurring, however, remains a formidable challenge.
Scanning tunnelling microscopy and femtosecond spectroscopy are among the most important developments in chemistry in recent decades. In the first – which led to the physics Nobel prize in 1986 – an atomically fine probe passes a tiny distance over a surface at a different electrical potential. The resulting ‘tunnelling current’ produces an image of the surface with atomic resolution. In the second – rewarded with the chemistry Nobel in 1999 – two laser pulses are spaced a few femtoseconds apart: the first excites the molecules to react, the second follows the progress of the reaction.
In 2016, researchers led by Jascha Repp and Rupert Huber of the University of Regensburg irradiated the tip of a scanning tunnelling microscope (STM) with an ultrashort pulse from a terahertz laser as it passed over a surface.2 They found the electromagnetic field of the pulse could interfere with the bias voltage sufficiently to extract a single electron from a pentacene molecule. By studying the effect on the tunnelling current, the researchers characterised the response of the molecule, combining the spatial resolution of scanning tunneling microscopy with the temporal resolution of femtosecond spectroscopy.
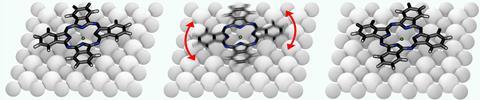
In the latest work, this team studied magnesium phthalocyanine – which has two mirror-symmetric stable states – adsorbed onto sodium chloride. When they positioned the STM tip over a magnesium phthalocyanine molecule and applied a light pulse of insufficient intensity to stimulate electron tunnelling, they found – as expected – that the molecule did not change state. However, about 10 picoseconds later, the researchers hit the molecule with a second pulse. This second pulse could induce switching. More surprisingly, they found that the probability that the molecule would switch when hit by the second pulse was increased by the first.
They concluded that the torsion from the electric field of the first pulse had caused the molecule to oscillate on the surface, and these oscillations in its electron density could influence the chemical reaction. ‘The first pulse can’t make it switch,’ explains Repp, ‘but it can make it more likely to do so once it’s promoted to a state in which it can.’ The researchers eventually hope to study chemical reaction mechanisms using the technique, but Repp says it will be difficult to find a suitable candidate. ‘This is one molecule,’ he says. ‘We would need to find a reaction in which the molecules could return to the same state at the end of every cycle.’
‘What’s fascinating here is not that the motions of atoms are controlled by a light pulse,’ says Ludwig Bartels at the University of California, Riverside. ‘What’s fascinating is that it’s controlled in a coherent way. For half a cycle, I have an electrical field pointing in one direction, and for half a cycle, I have a field pointing in the opposite direction, and depending on which way the field is pointing the reaction is either proceeding or not proceeding… Jascha and his colleagues achieved control of the smallest entity you can have in chemistry on the shortest timescale that makes sense in chemistry.’
References
1 D Peller et al, Nature, 2020, DOI: 10.1038/s41586-020-2620-2
2 T L Cocker et al, Nature, 2016, 539, 263 (DOI: 10.1038/nature19816)














No comments yet