Unstable white phosphorus seen joining up to make new 1D form on way to red allotrope of the element
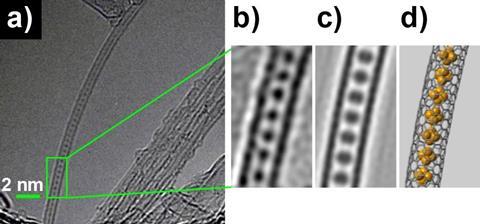
A new form of phosphorus made up of a chain of highly reactive white phosphorus molecules isolated inside single-walled carbon nanotubes has been discovered by UK scientists. Dubbed ‘pink phosphorus’, the one-dimensional polymeric structure offers a rare chance to study one of the most reactive and mysterious elements in the periodic table. The team was also given the chance to observe the first steps of the transformation from white phosphorus to the amorphous red allotrope of the element.
The PhD student who conducted the filling experiments [with white phosphorus], really needed nerves of steel for this
Christoph Salzmann, UCL
’The recent discovery of phosphorene, a single sheet of black phosphorus that is the 2D phosphorus analogue of graphene, has sparked a renewed interest in this highly diverse chemistry,’ says Christoph Salzmann at University College London, UK, who led the study. ‘Although the allotropy of elemental phosphorus is something that most chemists learn in school, the details of its structural chemistry remains poorly understood at both the experimental and computational level.’
White phosphorus is usually extremely hazardous to study because exposure to air turns it into a liquid that burns relentlessly. Meanwhile, the oxidation product combines with moisture from the air to produce highly corrosive phosphoric acid. Previous strategies for stabilising white phosphorus, which consists of tetrahedral P4 molecules, have used supramolecular cages but these could only contain a single P4 molecule.
However, Salzmann and his colleagues wanted to tame white phosphorus’ highly violent nature and work with it in larger amounts, so they set out to see if hollow carbon nanotubes were up to the job. But it was not a task for the faint hearted. ‘The greatest challenge was certainly handling molten white phosphorus,’ explains Salzmann. ‘Martin Hart, the PhD student who conducted the filling experiments, really needed nerves of steel for this – we could tell you some stories.’
Nano-test-tube
To fill the single-walled carbon nanotubes, they were first opened at their tips. These were then stirred in liquid white phosphorus before the excess white phosphorus was sublimed off leaving the phosphorus-filled tubes behind. Upon initial exposure to air, the oxidation reaction took place at the tips of the tubes, which the team thinks created a ‘cork’ that sealed the white phosphorus inside the tubes.
To conclusively prove the nanotubes had been filled and with high yields, they were characterised with a range of techniques including high resolution transmission electron microscopy which revealed the P4 molecules lined up inside the carbon nanotubes like peas in a pod. But there was an additional surprise. Upon exposure to the electron beam during electron microscopy measurements, the team observed that the P4 molecules start to link-up into chain structures resulting in the new pink form of the element.
‘Our study has provided a glimpse into the transformation from white to a polymeric form that can be thought of as “pink phosphorus” – on the way to the well-known amorphous red allotrope,’ explains Salzmann. ‘By confining the element within nanotubes we have created a simplified environment in which the individual steps in the conversion between allotropes can be studied at leisure.’
‘This is exciting for several reasons including a greater proportion by weight of phosphorus can be encapsulated by their method than is possible using other passivation methods studied to date,’ comments Jonathan Nitschke, who was one of the first people to isolate a single P4 molecule in a supramolecular cage in 2009. ‘I would also imagine that the electronic properties – conductivity and the like – of this conjugated chain of phosphorus atoms will be of interest, although perhaps difficult to study independently of the conductivity of the carbon sheath.’
The team is now investigating what structures and chemical behaviour would be observed by increasing the diameter of the carbon nanotubes. ‘Preliminary computational work has already highlighted that a multitude of different structures with complex chemical properties can be expected and we’ve just set out to chase them,’ says Salzmann. ‘And our fire extinguisher stands at the ready.’
References
M Hart et al, Angew. Chem., Int. Ed., 2017, DOI: 10.1002/anie.201703585







No comments yet