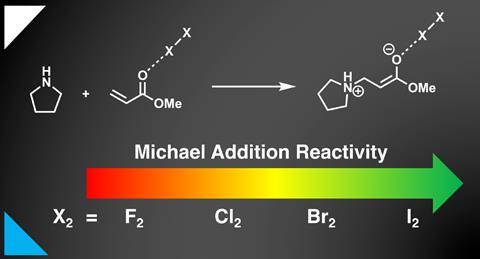
Iodine catalyses the Michael reaction – but not in the way chemists thought, a computational study has revealed. Rather than stabilising the reactants’ interactions through halogen bonding, reducing quantum mechanical Pauli repulsion is the secret of iodine’s success. ‘Our finding is a paradigm shift of looking at this reaction,’ says the study’s leader Matthias Bickelhaupt from the Free University Amsterdam in the Netherlands.
Discovered in 1887, the Michael addition is one of the most recognisable and useful reactions in organic synthesis. It creates a bond between an activated alkene, the Michael acceptor, and a carbon or heteroatom nucleophile, the Michael donor. The fact that iodine and other halogens act as catalysts for the reaction has been known for more than 100 years.
‘In the past, people have assumed that dihalogen molecules were able to activate the Michael acceptor the same way in which the Lewis acids do,’ says organic chemist Katherine Byrd who has published a review on Michael additions. Researchers assumed that by attaching itself to the Michael acceptor, iodine enhances orbital (HOMO–LUMO) interactions between acceptor and donor.
Investigating ‘a prototypical textbook aza-Michael addition’ between methyl acrylate and pyrrolidine, Bickelhaupt’s team has revealed what exactly gives iodine its catalytic superstar status: it reduces Pauli repulsion between acceptor and donor.
Pauli repulsion occurs between occupied orbitals, in this case between the pyrrolidine nitrogen’s lone electron pair and the acrylate’s π-electron system. With its low energy antibonding orbital, iodine pulls electron density away from the π system, decreasing Pauli repulsion to the donor orbital.
Computational dissection
Bickelhaupt and his collaborator Israel Fernández from Spain’s Complutense University of Madrid dissected the physical effects responsible for a smaller activation energy in the presence of iodine. To their surprise, the orbital interaction between donor and acceptor remained nearly constant.
‘Really the only reason why the interaction between nucleophile and iodine-complexed Michael acceptor gets stronger, more stabilising, is not because the orbital interactions get more stabilising, but because the closed shell–closed shell Pauli repulsion becomes less repulsive,’ Bickelhaupt explains.
‘I think it’s very interesting and it deserves some further investigation,’ says physical chemist Albeiro Restrepo Cossio from the University of Antioquia, Colombia. ‘I’m a theoretician, so I’m really pleased to see that there is a purely quantum effect, the Pauli repulsion, which has no classical analogue, that controls the reaction mechanism.’
But Cossio remains sceptical about how general the concept is. ‘I think they have too few cases; they only have one particular type of Michael reaction,’ he says. ‘I’m happy with the overall conclusions, but I have some reservations about the details.’
Bickelhaupt’s students are already investigating if halogens’ ability to reduce Pauli repulsion applies to other reactions. ‘I’m confident that this is a very widely acceptable new model for halogen-catalysed reactions,’ he says.
For organic chemists like Byrd, understanding the ins and outs of the Michael mechanism might help them design better catalysts. ‘Michael additions can be tricky depending on the substrates,’ she explains. ‘When you’re trying to do reactions in the lab, you are going to do whatever works. The real utility of this [study] will come in when things aren’t working.’
References
T A Hamlin, I Fernández and F M Bickelhaupt, Angew. Chem., Int. Ed., 2019, DOI: 10.1002/anie.201903196














No comments yet