Asymmetric reaction’s enantioselectivity turned on its head
Scientists have observed that switching the temperature of an asymmetric reaction from 0 to –44ºC inverts its enantioselectivity, adding to the intrigue of how homochirality originated in living organisms.
Nature almost always makes L-amino acids and D-sugars but scientists do not fully understand how this homochirality started. Asymmetric autocatalysis, a reaction where the chiral product catalyses its own formation, is thought to play a key part. A chiral trigger starts the reaction and determines the initial product’s chirality; autocatalysis then drives the resulting high enantiomeric excess.
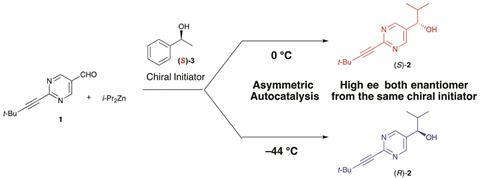
A new study, looking at the autocatalysis of pyrimidyl alkanol under various conditions, could steer us closer to the truth. Kenso Soai and colleagues at Tokyo University, Japan, and Merck Research Laboratories, US, found that the reaction’s products invert between the S and R configurations, in near-perfect enantiomeric excess, by adjusting the temperature from 0 to –44ºC, respectively. The team tested these reaction temperatures with different chiral amine and alcohol triggers and found that only temperature affected the chirality of the product.
Soai says ‘the important implication of the present results is that the chirality of the original trigger doesn’t necessarily determine the absolute configuration of the product.’ This unusual behaviour could indicate that the original chiral compound that led to all L-amino acids could have produced a D-amino acid excess under different conditions.
Ramon Rios Torres, an expert on asymmetric catalysis at Southampton University in the UK says the result is very interesting and raises some important questions around preconceived ideas that chemists have regarding reaction mechanisms. ‘If this could be applied to different reactions it would be of great interest because with the same catalyst we can achieve both enantiomers of a reaction, but this is very far away,’ he adds.
The mechanism behind this temperature control is not clear, but the team speculates that it may be down to aggregation as the reaction proceeds. They continue to investigate how the enantioselectivity is inverted.
References
This paper is open access
A Matusmoto et al, Org. Biomol. Chem., 2016, DOI: 10.1039/c6ob02415g











No comments yet