The use of light-absorbing chiral iminium ions enables novel syntheses
Researchers have created a new class of organic catalysts that mimic the way vertebrate vision works when light excites iminium ions, unlocking previously inaccessible reaction pathways which allow stereochemical control of the end product. The approach could lead to novel catalytic frameworks for enantioselective photochemistry and chiral molecule synthesis, which could be particularly useful for finding new drug molecules.
Using chiral iminium ions in organocatalysis is nothing new. For the past fifteen years they’ve been important intermediates in reactions that synthesise enantioenriched chiral molecules. This catalytic platform has been exclusively used within polar, ground state reactivities. But Paolo Melchiorre and his colleagues at the Institute of Chemical Research of Catalonia (ICIQ), Spain, decided to explore the reactivity of iminium ions in an excited state. ‘We demonstrated that the synthetic potential of chiral iminium ions is not limited to the ground-state domain, but can be further expanded by exploiting its photochemical activity,’ says Melchiorre.
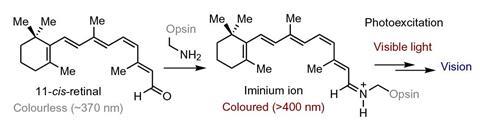
It was already known that iminium ions have light-absorbing properties, particularly for their pivotal biochemical role in converting light into electrical signals to enable vertebrate vision. But Melchiorre’s team took inspiration following an observation in the lab. When mixing an amine catalyst with an aromatic enal the solution turned from colourless to yellow. This indicated that the transiently generated iminium ions absorbed visible light, which is essential to reach an electronically excited state.
Having recently demonstrated that chiral enamines could directly participate in the photoexcitation of substrates while inducing the stereocontrolled formation of chiral products, the team inferred that photoexcited iminium ions could function as a strong oxidant and lead to new reactivities. To that end, the researchers made organocatalysts based on the amino acid proline using a commercially available diarylprolinol silylether catalyst, which is generally used in stereoselective iminium ion-catalysed thermal reactions.
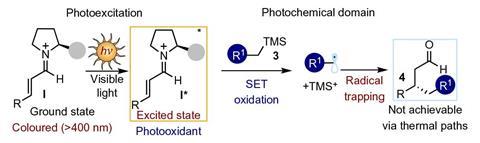
However, their catalysts were designed to produce a colour change to know when transient iminium ions are formed during the reaction between the catalysts and substrate. A single violet LED was then shone to take the iminium ions to an excited state and trigger unique stereocontrolled mechanisms. Turning off the light shut down the reactions.
‘This is a really exciting paper,’ comments David MacMillan, pioneer of iminium ion-mediated catalysis at Princeton University, US. ‘It’s not only a conceptually unique transformation, in many ways it opens the doors to many reactions via this new platform of excited iminium activation. Very cool stuff!’
Tehshik Yoon who investigates photocatalysis at the University of Wisconsin-Madison, US also thinks highly of the work. ‘One can imagine that this concept might be applied not only to the conjugative addition reactions reported here but to an entire family of related photoreactions.’
’We believe that combining enantioselective organocatalysis with photochemistry can offer unconventional ways of making chiral molecules,’ says Melchiorre. ‘We expect that this approach will not be limited to organocatalysis, but could be applied to other areas of modern synthetic chemistry,’ he adds.
References
M. Silvi, et al., Nat Chem, 2017, DOI: 10.1038/nchem.2748










No comments yet