(−)-Novofumigatonin
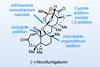
Oxidations abound in this satisfying synthesis, with a delicate nitrile hydrolysis to finish
There’s an old joke among alkaloid chemists that each atom of nitrogen in a natural product target will add a year to your PhD. I haven’t heard such an axiom for oxygen, but I imagine that plotting the number of atoms against difficulty gives more of a bell curve. Having none certainly makes things very hard, as so many disconnections revolve around the carbonyl group.