(+)-Pleuromutilin
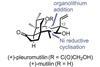
Total synthesis is sometimes the only way to explore the chemical space around a natural product
It often feels that chemists’ talk of biological activity at the start of total synthesis papers is just boilerplate. But natural products continue to be an important source of new drugs. In therapeutic areas such as antibiotics and oncology, natural products and their derivatives make up more than half of approved drugs.1 Big Pharma’s enthusiasm for natural product chemistry has waxed and waned over the years, but with the spectre of a post-antibiotic era looming, chemists are again looking to nature for inspiration in the fight against drug-resistant bacteria.