Chris Smith
This week the chemical cause of transatlantic linguistic friction. Is it an um or an ium at the end? It turns out us Brits might have egg on our faces as well as a liberal smattering of what we call aluminium.
Kira J. Weissman
'I feel like I'm trapped in a tin box at 39000 feet'. It's a common refrain of the flying-phobic, but maybe they would find comfort in knowing that the box is actually made of aluminium – more than 66000 kg of it, if they're sitting in a jumbo jet. While lamenting one's presence in an 'aluminium box' doesn't have quite the same ring, there are several good reasons to appreciate this choice of material.

Pure aluminium is soft. However, alloying it with elements such as such as copper, magnesium, and zinc, dramatically boosts its strength while leaving it lightweight, obviously an asset when fighting against gravity. The resulting alloys, sometimes more malleable than aluminium itself, can be moulded into a variety of shapes, including the aerodynamic arc of a plane's wings, or its tubular fuselage. And whereas iron rusts away when exposed to the elements, aluminium forms a microscopically thin oxide layer, protecting its surface from further corrosion. With this hefty CV, it's not surprising to find aluminium in many other vehicles, including ships, cars, trucks, trains and bicycles.

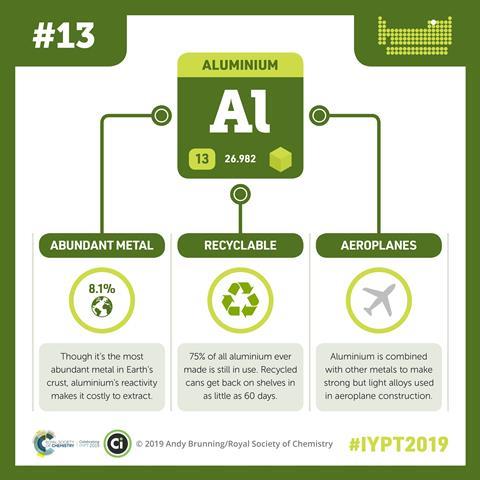
Happily for the transportation industry, nature has blessed us with vast quantities of aluminium. The most abundant metal in the Earth's crust, it's literally everywhere. Yet aluminium remained undiscovered until 1808, as it's bound up with oxygen and silicon into hundreds of different minerals, never appearing naturally in its metallic form. Sir Humphrey Davy, the Cornish chemist who discovered the metal, called it 'aluminum', after one of its source compounds, alum. Shortly after, however, the International Union of Pure and Applied Chemistry (or Iupac) stepped in, standardizing the suffix to the more conventional 'ium'. In a further twist to the nomenclature story, the American Chemical Society resurrected the original spelling in 1925, and so ironically it is the Americans and not the British that pronounce the element's name as Davy intended.
In 1825, the honour of isolating aluminium for the first time fell to the Danish Scientist Hans Christian Ørsted. He reportedly said of his prize, 'It forms a lump of metal that resembles tin in colour and sheen' – not an overly flattering description, but possibly an explanation for airline passengers' present confusion. The difficulty of ripping aluminium from its oxides – for all early processes yielded only kilogram quantities at best – ensured its temporary status as a precious metal, more valuable even than gold. In fact, an aluminium bar held pride of place alongside the Crown Jewels at the 1855 Paris Exhibition, while Napoleon is said to have reserved aluminium tableware for only his most honoured guests.



It wasn't until 1886 that Charles Martin Hall, an uncommonly dogged, amateur scientist of 22, developed the first economic means for extracting aluminium. Working in a woodshed with his older sister as assistant, he dissolved aluminium oxide in a bath of molten sodium hexafluoroaluminate (more commonly known as 'cryolite'), and then pried the aluminium and oxygen apart using a strong electrical current. Remarkably, another 22 year-old, the Frenchman Paul Louis Toussaint Héroult, discovered exactly the same electrolytic technique at almost exactly the same time, provoking a transatlantic patent race. Their legacy, enshrined as the Hall–Héroult process, remains the primary method for producing aluminium on a commercial scale - currently million of tons every year from aluminium's most plentiful ore, bauxite.

It wasn't only the transportation industry that grasped aluminium's advantages. By the early 1900s, aluminium had already supplanted copper in electrical power lines, its flexibility, light weight and low cost more than compensating for its poorer conductivity. Aluminium alloys are a construction favourite, finding use in cladding, windows, gutters, door frames and roofing, but are just as likely to turn up inside the home: in appliances, pots and pans, utensils, TV aerials, and furniture. As a thin foil, aluminium is a packaging material par excellence, flexible and durable, impermeable to water, and resistant to chemical attack – in short, ideal for protecting a life-saving medication or your favourite candy bar. But perhaps aluminium's most recognizable incarnation is the aluminium beverage can, hundreds of billions of which are produced annually. Each can's naturally glossy surface makes as an attractive backdrop for the product name, and while its thin walls can withstand up to 90 pounds of pressure per square inch (three times that in a typical car tyre), the contents can be easily accessed with a simple pull on the tab. And although aluminium refining gobbles up a large chunk of global electricity, aluminium cans can be recycled economically and repeatedly, each time saving almost 95% of the energy required to smelt the metal in the first place.

There is, however, a darker side to this shiny metal. Despite its abundance in nature, aluminium is not known to serve any useful purpose for living cells. Yet in its soluble, +3 form, aluminium is toxic to plants. Release of Al3+ from its minerals is accelerated in the acidic soils which comprise almost half of arable land on the planet, making aluminium a major culprit in reducing crop yields. Humans don't require aluminium, and yet it enters our bodies every day – it's in the air we breathe, the water we drink, and the food we eat. While small amounts of aluminium are normally present in foods, we are responsible for the major sources of dietary aluminium: food additives, such as leavening, emulsifying and colouring agents. Swallowing over-the-counter antacids can raise intake levels by several thousand-fold. And many of us apply aluminium-containing deodorants directly to our skin every day. What's worrying about all this is that several studies have implicated aluminium as a risk factor for both breast cancer and Alzheimer's disease. While most experts remain unconvinced by the evidence, aluminium at high concentrations is a proven neurotoxin, primarily effecting bone and brain. So, until more research is done, the jury will remain out. Now, perhaps that IS something to trouble your mind on your next long haul flight.
Chris Smith
Researcher Kira Weissman from Saarland University in Saarbruken, Germany with the story of aluminium and why I haven't been saying it in the way that Humphrey David intended. Next week, talking of the way the elements sound, what about this one.
Brian Clegg
There aren't many elements with names that are onomatopoeic. Say oxygen or iodine and there is no clue in the sound of the word to the nature of the element, but zinc is different – zinc, zinc, zinc, you can almost hear a set of coins falling into an old fashioned bath. It just has to be a hard metal. In use, zinc is often hidden away, almost secretive. It stops iron rusting, sooths sunburn, keeps dandruff at bay, combines with copper to make a very familiar gold coloured alloy and keeps us alive but we hardly notice it.
Chris Smith
And you can catch up with the clink of zinc with Brian Clegg on next week's Chemistry in its element. I'm Chris Smith, thank you for listening and goodbye.













No comments yet