Ben Valsler
This week, what does a nightclub dancefloor have to do with a biology lab? Kat Arney explains…
Kat Arney
In the cold light of day, fluorescein doesn’t look like much – just a dull orangey-red powder. But like a party animal whose true colours only show after a few drinks and a spin on a nightclub dancefloor, dissolve it in alcohol or water and put it under an ultraviolet light and fluorescein comes alive, glowing with an eerie green hue.
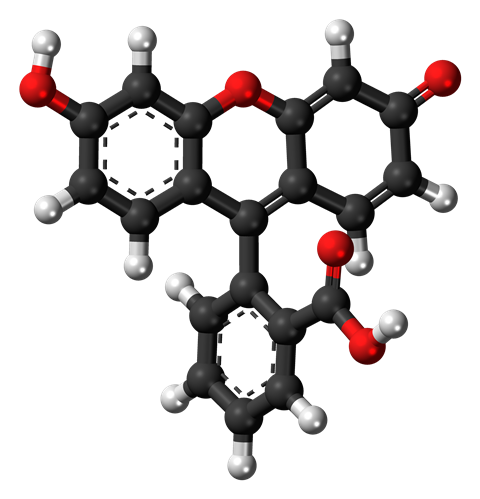
An organic molecule made solely of carbon, oxygen and hydrogen, its fluorescent properties come from the fact that it’s made up for four hexagonal rings of carbon joined together. UV light gets the electrons in these carbon rings overexcited and they start dancing about – just like our disco-loving partygoer – releasing their excess energy as light.
Similar in its properties to naturally-occurring fluorescent molecules made by certain species of bacteria, fluorescein was first artificially synthesised by Nobel prize-winning German chemist and dye wizard Adolf von Baeyer in 1871. Since then, it’s been widely used in all kinds of applications ranging from lab research to healthcare, oilfields to air-sea rescue.

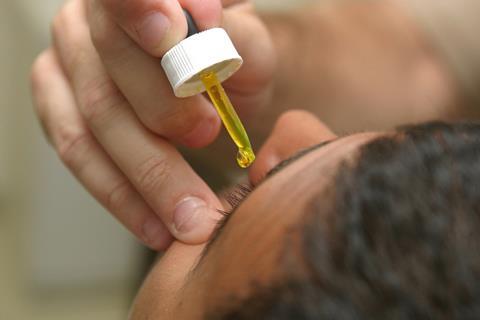
In fact, if you’re a contact lens wearer, you’ve probably had fluorescein – or at least its salt, fluorescein sodium – put in your eyes when you go for your checkup at the optician, usually by gently touching a piece of blotting paper steeped in the stuff against your eye. What they’re looking for are tiny scratches on the surface of the eyeball. And because fluorescein pools in these marks, they show up as green traces when seen under blue light (UV isn’t used here, because it’s too damaging to your vision).
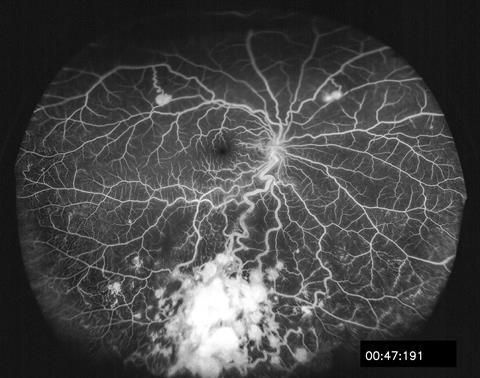
Other medical uses include using fluorescein injections into the bloodstream to trace blood vessels – a technique known as a fluorescein angiogram, first developed in 1959 by a couple of American medical students, Harold Novotny and David Alvis. They were working on ways to take photographs of the blood vessels at the back of the eye, and noticed that their camera was picking up slight fluorescence from the crystalline compounds that make up the lens. Figuring that putting a fluorescent dye in the blood would prove to a be a good way to highlight the delicate network of vessels, they set about testing various combinations of dyes and filters until they landed on the ones that are still in use around the world today.
What’s more, fluorescein is a highly social chemical, easily attaching itself to other biological molecules such as antibodies or the building blocks of DNA. And it’s because of this property that it has become is beloved of biologists. A version of fluorescein called FITC, or ‘fitsy’ as it’s usually pronounced, is commonly used to stain whole cells or the molecules inside them. This enables researchers to visualise them through a microscope equipped with UV light or lasers, creating intricate images of the inner workings of cells. FITC- or fluorescein-labelled antibodies are often used in automated cell sorting machines, alongside other fluorescent markers that glow with different colours, to separate out cells with distinct characteristics so they can be studied in more depth.

It’s fluorescein’s ability to dissolve easily in water that also makes it suitable for applications on a much larger scale. Because there’s a certain amount of UV in the sun’s rays, it will glow green in regular sunlight, as well as under the beams of a UV lamp. So it’s used to trace the flow of water in drains and other water courses, as well as highlighting leaks of nasty stuff like sewage. In world war two, Nazi planes were equipped with fluorescein ‘flares’ – small packages of the chemical that would be released if the plane crashed into water – revealing the location of the wreckage. In the 1960s, spacecraft splashing down in the ocean released fluorescein tracers, enabling rescuers to spot the craft – and its astronaut occupants – bobbing on the waves.

Finally, a hundred pounds of fluorescein was first used to turn the Chicago river green to celebrate St Patrick’s Day back in the 1960s, but it was quickly replaced with more eco-friendly vegetable-based dyes following a campaign by environmentalists. Less fun, perhaps, but kinder to the local wildlife, to be sure.
Ben Valsler
Kat Arney on fluorescein, a compound that helps us see, and be seen. Next week, pack your bags, we’re going on holiday with Emma Stoye…
Emma Stoye
One of the most annoying things about going on holiday – up there with trying to cram everything into your hand luggage, or worrying about where you left your passport – is the prospect of being constantly tormented by bugs. If like me you have pale skin – an irresistible lure for flies, ticks and mosquitoes – one of the standard scents of overseas travel is the lemony tang of insect repellent.
Ben Valsler
Find out how Emma avoids biting bugs next time. Until then, drop us a line with any suggestions for compounds to cover by tweeting @chemistryworld. I’m Ben Valsler, and I’ll be back next week.













No comments yet