To some, 'click chemistry' is simply a relabelling of standard organic chemistry practices. Others follow its principles almost religiously
When chemistry is compared to cookery, that’s not just because it involves mixing the ingredients and baking them, but also because you can’t always be sure what will come out of the oven. Your chemical soufflé may have sunk, or gone black, or separated. Or more often than not, you get a bit of soufflé mixed with a lot of other unappetising junk. Chemistry just isn’t as reliable as we’d like.

That’s what led Nobel laureate Barry Sharpless of the Scripps Research Institute in La Jolla, California, US, several years ago to advocate a new approach to making molecules, called click chemistry. If you prepare all your recipes using so-called ’click’ reactions, say its advocates, you’ll get perfect results every time.
But trying to define click chemistry brings to mind the blind Indian sages trying to describe an elephant. Since being coined by Sharpless, it now seems to mean many different things to different people. Some will say it is a certain type of copper-catalysed reaction for making organic molecules, others a general new way of making polymers. Some think it has something to do with enzyme catalysis, others that it is a new approach to organic synthesis in general.
Some even question whether it exists at all. ’Click chemistry is neither a concept nor a discovery,’ said one organic chemist I approached. ’It is simply the collection of reactions that emerges upon cataloguing the available synthetic reactions.’ And if all it implies is that chemists should look for efficient reactions, he adds: ’That is obvious and does not constitute an idea - it is merely common sense’. Others are even more dismissive, suggesting that the notion of ’click chemistry’ is positively misleading. ’Like many other chemists, I regret the use of this meaningless verbiage in synthetic chemistry,’ said one. ’It is an unneeded and unwelcome publicity stunt.’
’"Common sense" is indeed the best definition of click chemistry,’ counters Valery Fokin, Sharpless’s colleague at Scripps. ’Many of us somehow forget common sense when we’re trying to make something useful, so it is worth reminding ourselves of what it can accomplish.’
So is click chemistry a useful concept or not? What did Sharpless have in mind when he introduced the idea, and why did he consider it necessary? Why, if click chemistry is so roundly criticised by some chemists, does it now feature in at least 600 papers, with more being added to the list almost daily? Why has it become so protean, if not indeed misunderstood?
Bowing to nature
Sharpless, who was awarded his Nobel in 2001 for developing chiral catalysts for oxidation reactions in organic chemistry, is among the many organic chemists who have been struck by how cumbersome and tortuous our efforts at making carbon-based molecules are in comparison with nature’s (see Chemistry in Britain, November 2001, p26). Why do we find it so difficult?
Well, for one thing, nature’s chemists are enzymes that work at the atomic scale - whereas we can’t. ’In the "unnatural" process it is our hands controlling the tools,’ says Sharpless. ’Is it any wonder that things rarely work as planned? No matter how clever the brain behind the hands, they remain ludicrously out of scale for the manipulations intended.’
But he thinks there are also problems with what we’re trying to make. Since organic molecules generally have frameworks of carbon atoms, one of the biggest challenges in chemical synthesis is finding ways to link two carbon atoms together to make C–C bonds. In nature this often happens via a generic reaction called the aldol condensation, which involves carbonyl (C=O) groups. Sharpless feels that organic chemists have long made the error of thinking that they should mimic nature in finding ways of creating new C–C bonds. But most synthetic reactions that exist to do this have only a very modest thermodynamic driving force - which means that they happen inefficiently, giving low yields.
That is a huge burden for pharmaceutical chemists, because every low-yield step in a multi-step synthesis of some biologically active molecule slashes the final yield of product and makes the synthesis wasteful and costly. It also makes the process of drug discovery slow, because it is expensive in labour, time and materials to make new candidate drugs.
So Sharpless argues that, rather than slogging away at what we do badly, we should focus on what we can do well. Nature’s chemistry isn’t just about making C–C bonds, he says; in fact, the small-molecule building blocks of nature’s key compounds - proteins, nucleic acids and polysaccharides - generally have no more than six consecutive C–C bonds. They are full of heteroatom bonds, in which carbon is linked to atoms of a different element, mostly oxygen or nitrogen. The heteroatom X often bridges two carbons, creating C–X–C units.
Linkages of this sort, he says, are much easier to make efficiently. So why not focus on them? In other words, we can start with molecules that have all the C–C bonds we need, and think about how to link up these reagents in the right way via heteroatom bonds made using efficient reactions. This, he says, turns the philosophy of drug development on its head. Rather than choosing some daunting molecular target and then slaving at the bench for years to try to make it, we should pay greater attention to molecules that we know how to make, using reliable and effective heteroatom chemistry.
Click sense
What, though, are these ’reliable reactions’? That’s where click chemistry comes in. Sharpless and other converts to the ’click’ concept have concentrated on finding reactions with a large thermodynamic driving force, which give virtually complete conversion of reagents to a single product. Such reactions are ’spring-loaded’ - they start with ’high-energy’ compounds that will ’click’ together in a predictable way with very little prompting. Ideally, such reactions should proceed under relatively mild reaction conditions - no intensive cooking, which runs the risk of breaking down some reagents or products into unwanted byproducts. And they should work in solvents that are benign, especially water. All of this potentially makes click chemistry both clean (it doesn’t produce anything you don’t want) and green.

’The lack of byproducts and quantitative yields are the big advantages of click chemistry for materials chemistry,’ says polymer chemist Craig Hawker of the University of California in Santa Barbara, US. ’This allows multiple functionalisation along a polymer backbone to be accomplished with high efficiency, and the products isolated easily.’ M G Finn, a collaborator with Sharpless and Fokin at Scripps on the development and use of click reactions, says that materials scientists picked up on the ideas of click chemistry much faster than many organic chemists. ’This is not surprising, since Barry and the rest of us had polymer chemistry firmly in mind when we were thinking about the subject,’ he says. ’It’s the one area of synthesis in which function is always far more important than structure as a criterion for evaluation.’
Hawker has no doubts that the ’click’ perspective was valuable for his own efforts to make new, complex polymers. Among those he has aimed to make are block copolymers, in which two or more chains of different polymers are stitched together. These materials can combine the properties of different polymers that, in pure form, don’t mix readily. For example, the rubbery material used in some training-shoe soles is a triblock copolymer of polystyrene and polybutadiene. The chain segments tend to segregate into different domains at the microscopic scale, and this microstructure can give the material useful properties. ’I have been searching for years for a reaction that allows me to couple together high molecular weight polymers to give block copolymers,’ says Hawker. ’With click chemistry this can now be achieved.’
Hawker and others have also used click reactions to put polymers together in architectures more complex than conventional chains, such as the tree-like molecules called dendrimers, which have uses ranging from catalysis and adhesion to drug delivery. For example, his team has used click chemistry to make polymer nanoparticles with chemically active surfaces. They prepared polymer micelles (spherical aggregates) from chains primed for click chemistry, and then used small molecules with the conjugate ’click’ group simultaneously to crosslink the chains and to introduce new reactive ’click’ groups where other molecular substituents could be plugged into the particles.
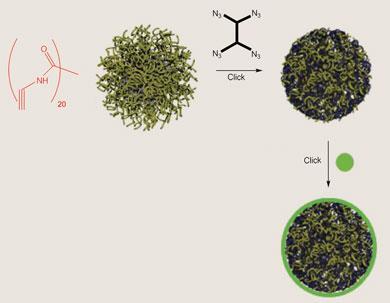
Cross reactions
You can hardly expect to suggest that synthetic organic chemists have been barking up the wrong tree or conducting sloppy, ineffectual chemistry without making some of them cross. What some seem to object to is Sharpless’s coining of a new term to describe any reaction that is efficient by virtue of its thermodynamic impetus - and the concomitant implication that until now organic chemists have neglected to select their reactions on such grounds. ’Of course we use known and efficient reactions in our work, just like everyone else,’ says one critic. ’But we don’t call them "click".’
Harvard chemist George Whitesides, however, concurs with the idea that today’s organic synthesis is rarely a paradigm of efficiency. Most of the reactions described in text books, he says, are actually too messy to be of much practical value, and chemists typically have to make do with only a small subset of them. He feels that click chemistry has the virtue of making that shortcoming explicit.
Finn recognises that not everyone will be ready to abandon methods that don’t reach the click chemistry standards of reliability. ’A strict adherence to the "click" principle of using of the most reliable synthetic techniques is harder than it sounds for most of us,’ he says, ’since it forces us to abandon many of those cherished reactions on which we cut our teeth.’ He adds that ’many of the reactions that we all love are not "click" reactions, not because they aren’t beautiful or useful or interesting, but just because they cannot be applied to a wide range of substrates under a wide range of conditions’.

Sharpless says that in fact chemists thought more in ’click’ terms 100 to 50 years ago than they do now. This was a matter of necessity - they had few catalysts, but had to rely instead only on heat to speed reactions up. And the choice of solvents and of purification techniques was much smaller. As a result, they would have got nowhere at all with organic synthesis unless they selected those reactions that could be relied on to give a single product in good yield that was easy to isolate and purify.
Yet neither do some chemists take kindly to the idea that they should focus on what is easy to make. To some, the challenge of difficult synthetic targets has always been a driver for discovering new synthetic techniques. ’To suggest that the synthetic chemists should rest on their laurels and restrict themselves to those reactions and those molecules that they know how to perform and synthesise is absurd,’ says one. ’Based on such philosophy we would neither have any progress in the art of chemical synthesis nor would we have witnessed the last two Nobel Prizes for the field [2001 and 2005].’ The former is Sharpless’s own award.
All the same, Sharpless and his colleagues insist that synthetic chemistry can only benefit from identifying the most powerful transformations that operate under the simplest conditions, and seeing what can be made with them. One of the ’click’ reactions they have particularly championed is a form of cycloaddition typified by the so-called Huisgen reaction, in which alkyne groups (carbon-carbon triple bonds) dangling from the end of a molecule are combined with azides (N3 groups, containing two nitrogen-nitrogen double bonds) to make heteroatoms called 1,2,3-triazoles in a regioselective manner. In 2002, Sharpless and colleagues at Scripps, and Morten Meldal and coworkers at the Carlsberg Laboratory in Denmark, independently showed that when the reaction is catalysed with a copper( I ) salt, it links the two carbons in the acetylene group to different nitrogens in the azide, generating a five-membered ring in an extremely efficient manner, giving very high yields. ’This improved version is a very useful reaction for joining chemical building blocks together to form larger assemblies and conjugates,’ says K C Nicolaou, a synthetic organic chemist also at Scripps.
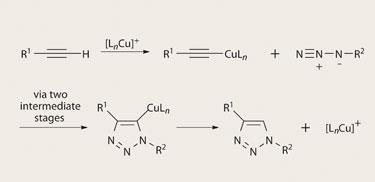
The particular attraction of this reaction is that, although both acetylene and azide groups are ’high-energy’, potentially reactive components, they aren’t recognised as such by biological molecules. So the reaction can be conducted cleanly in the presence of, say, enzymes without reacting with everything in sight. That is one of the most desirable attributes of click chemistry (but one that is rarely achieved): the reactions should be ’orthogonal’ to a wide range of other functional groups. The aim, says Sharpless, is to create highly reactive ’sticky spots’ with the right target groups while having them remain invisible to most other types of molecule.
This orthogonality meant that Sharpless and his coworkers could use the reaction to assemble molecules in situ inside an enzyme’s active site - a process that sounds rather like putting a firecracker in your mouth. Small molecules that inhibit enzymes by slotting into their ’jaws’ and blocking access to the enzyme’s usual target have many pharmacological uses. Sharpless’s team has been using click chemistry to make such inhibitors from libraries of small building blocks primed with acetylene and azide substituents, which will bind in the active site. The idea is that the active site acts as a mould to create molecules that make a good fit. In 2001 the Scripps researchers used this approach to construct molecules that would fit into the active site of acetylcholinesterase, an enzyme involved in neurotransmission. Along with their colleague Hartmuth Kolb, Sharpless and Fokin have used the same idea to create inhibitors of carbonic anhydrase, an enzyme targeted for treating glaucoma, and HIV protease, an enzyme without which HIV transmission can’t occur. The molecules they identified this way were at least as effective in binding to the enzymes as those currently used as drugs.
The copper-catalysed Huisgen reaction has become so popular that it is now almost synonymous with click chemistry, and it has been put to all kinds of uses. For example, Benjamin Cravatt at Scripps, has used it to fish out of labelled proteins from complex mixtures produced by living cells. Hawker has welcomed the azide-acetylene click too. ’In my own work, it is the orthogonality and efficiency of this reaction which has allowed us to make well-defined, multifunctional materials with a precision approaching small molecule chemistry,’ he says.
Albert Eschenmoser, an organic chemist at the ETH in Zurich, Switzerland, feels that the discovery was pivotal in persuading other chemists to take click chemistry seriously. It was, he says, ’an improvement in the efficiency and scope of a known organic reaction that was so dramatic that it clearly amounted to a discovery of the first order in the field of synthetic methodology, one whose applicability turned out to go far beyond organic chemistry.’ And he admits that the discovery silenced his own scepticism about the field. ’I stopped laughing at the term click chemistry when I realised how the psychology and research attitude standing behind this "joke of a term" can lead to a discovery of such importance.’
Chemical tool box
But click chemistry needs more than a single trick. Sharpless and his colleagues have been gradually adding others to the list of reactions that fit the click criteria - among them, reactions that open ’spring-loaded’ small rings with a wide variety of attacking groups. ’My hope is that click chemistry develops a large enough toolbox to allow a wide range of functional groups to be prepared,’ says Hawker. ’Then a multi-step synthesis would employ two or three click reactions as well as standard chemical transformations. I see it as a major aid to synthesis rather than a synthetic philosophy unto itself.’
Finn, meanwhile, hopes that the click philosophy will enable chemistry to be done faster and more effectively in places that have previously lacked the facilities for complex synthesis. ’We nurture a fantasy that people in developing countries could someday make pharmaceuticals or pesticides on the spot, not in the form of a kit but in a real discovery mode,’ he says. ’We also believe that a ready supply of click building blocks and coupling methods may be useful in emergency situations such as the need for a new antiviral agent to respond to an outbreak of disease.’
Others will no doubt continue to resist the idea. ’Chemists have always used their common sense to choose the most efficient reactions to synthesise their favourite molecules,’ argues one organic chemist. ’We do not need to invent a new name for such reactions. Rather, we need more of those reactions! Synthetic chemists interested in providing a substance in the most economical and efficient way will always seek to apply the most efficient reactions, whether we call them "click" or not.’
Finn doesn’t disagree with that, but he also sees the value of ’click’ as a way of sharpening the critical faculties of chemists when they come to plan their syntheses. ’If click chemistry comes to be recognised as a shorthand way of saying that you’re using one or a few particularly good reactions because they improve your chances to achieve your target function, that’s fine. Also, if people start to ask whether or not a reaction has reached "click" status, that would be great, since it would embody an awareness of what makes reactions truly useful in a practical way.’
It’s obvious, but true nonetheless, to say that the concept either clicks with you or it doesn’t.
Philip Ball is a science writer based in London
Further reading
C J Hawker and K L Wooley, Science, 2005, 309, 1200 (DOI: https://doi.org/10.1126/science.1109778)
R K O’Reilly et al, Chem Soc Rev., 2006, 35, 1068 (DOI: https://doi.org/10.1039/B514858H)
V V Rostovstev et al, Angew. Chem. Int. Ed., 2002, 41, 2596 (DOI: https://doi.org/10.1002/1521-3773(20020715)41:14%3C2596::AID-ANIE2596%3E3.0.CO;2-4)
H C Kolb et al, Angew. Chem. Int. Ed., 2001, 40, 2004 (DOI: https://doi.org/10.1002/1521-3773(20010601)40:11%3C2004::AID-ANIE2004%3E3.0.CO;2-5)












No comments yet