Mike Sutton reports on Glenn Seaborg's adventures among the actinides
The discoverer of a previously unknown chemical element immediately joins a very small group of distinguished scientists. To identify more than one is an even rarer accomplishment. Marie and Pierre Curie found two, Jons Jacob Berzelius four and Humphry Davy six – but so far only one investigator has been involved in adding 10 to the list. In 1997 this achievement was recognised by the naming of element number 106 as Seaborgium, after its co-discoverer Glenn Seaborg whose centenary is celebrated this year. Others – including Albert Einstein and Dmitri Mendeleev – received this honour posthumously, but as yet only Seaborg has earned it during his lifetime.

He had an eventful life. Besides making discoveries which earned him a Nobel prize in chemistry, Seaborg helped to develop the nuclear weapons that ended the second world war. Afterwards, he worked on peaceful uses for atomic energy. He held several senior university appointments, supervised 68 PhD students, authored or coauthored over 500 scientific papers and was a scientific adviser to 10 US presidents. Meanwhile, he had a busy family life and pursued some strenuous hobbies – he was clearly a genius at time-management!
The early years
Glenn Theodore Seaborg was born on 19 April 1912 in Ishpeming, an iron-mining town in Michigan, US. His mother had migrated to the US from Sweden, as had both his father’s parents, and Seaborg spoke Swedish before learning English. In 1922, seeking better employment and educational opportunities, the family moved to a small Californian community called Home Gardens – now a suburb of Los Angeles.
The Seaborgs endured hard times during the 1920s and 1930s, as Glenn’s father – a mechanic – was frequently unemployed. Nevertheless, Seaborg completed a chemistry degree at the University of California, Los Angeles in 1933, financing his studies with several part time jobs. After another year taking postgraduate physics courses he moved to the University of California, Berkeley, gaining a PhD there in 1937. Berkeley’s dean of chemistry, Gilbert Lewis, hired him as a laboratory assistant and they published numerous papers together.
By day I ran experiments on acids and bases as the personal assistant of Gilbert Lewis. And by night I spent my free time exploring the mysteries of the atom
Glenn Seaborg
In 1939 Seaborg became an instructor at Berkeley, and might have proceeded to a conventional career in mainstream chemistry but for two momentous events which occurred in that year – the discovery of nuclear fission and the start of the second world war. By then, he had already become informally acquainted with one of the world’s most sophisticated scientific tools – the cyclotron. Its principles had been outlined by Leo Szilard in the 1920s, but it was Ernest Lawrence – assisted by Milton Livingston – who constructed the first working instrument at Berkeley in 1931.
It used high-frequency alternating electric currents to accelerate charged particles on a spiral path until they reached unprecedented velocities. When beamed at suitable targets these particles generated many previously unknown isotopes. Several had valuable applications – for example iodine-131 was used successfully to treat thyroid cancer.
However, Seaborg’s introduction to this advanced research programme was strictly unofficial. He later recalled: ’By day I ran experiments on acids and bases as the personal assistant of cigar-chewing Gilbert Lewis, the world’s pre-eminent physical chemist. And by night I spent my free time exploring the mysteries of the atom. My career started with a lucky encounter when a physicist with access to the coveted cyclotron handed me a "hot" target and asked me to identify chemically the radioisotopes it contained. At a small workbench, with tap water, a sink, a fume hood and supplies bootlegged from the chemistry department, I performed the separation – and demonstrated the usefulness of a chemist in this area dominated by physicists. That success opened the door to a collaboration that steered me into my life’s work.’
Exciting news reached Berkeley from Europe in 1939. In the previous December, Otto Hahn and Fritz Strassmann had performed the first successful nuclear fission experiment in Berlin, Germany, without fully appreciating what they had achieved. In January 1939, their result was correctly interpreted by Hahn’s former colleague Lise Meitner – by then a refugee in Sweden – and her nephew, Otto Frisch. On hearing this, Berkeley-based Edwin McMillan (who was to share the 1951 Nobel prize in chemistry with Seaborg) intensified his efforts. In May 1940, his team produced tiny amounts of the first artificial element – number 93 – by bombarding uranium with neutrons.
The war years
While war raged in Europe, the US – though still neutral – was increasing its military capability. McMillan left Berkeley to work on radar for the government in November 1940, but encouraged Seaborg to continue his nuclear research. Early in 1941, Seaborg and his assistants confirmed the identity of another artificial element – number 94 – produced by bombarding uranium with deuterons. Since the new elements followed uranium in the periodic table they were assigned the names neptunium and plutonium, but their discovery remained unpublished.
Secrecy was imperative because one isotope (plutonium-239) underwent spontaneous fission with the release of neutrons, making possible a chain reaction which could generate colossal amounts of energy. The manufacture of nuclear weapons was already under consideration – senior physicists led by Einstein had alerted President Franklin Roosevelt to their potential impact in August 1939. When Japan’s attack on Pearl Harbour in Hawaii brought the US into the war in December 1941, a research and development programme – soon to be code-named the Manhattan Project – was up and running. In June 1942, Seaborg left Berkeley to join it, pausing on the way to get married.

Helen Griggs worked at Berkeley as Lawrence’s secretary, and she and Seaborg had been dating since 1941. They took the train to Chicago together, and were married during a short stopover in Nevada. Though the ceremony was brief and austere, the marriage was long and happy, enduring until Seaborg’s death 56 years later. The couple had six children and shared numerous enthusiasms – particularly hiking along wilderness trails. Seaborg often described Helen as ’my best discovery’.
The newlyweds reached Chicago as the Manhattan Project was approaching a critical point. In December 1942, the world’s first controlled nuclear chain reaction began, inside a pile of uranium and graphite blocks which Enrico Fermi had assembled in a disused sports stadium. The race for the bomb was on. Fermi’s multinational team – some of them refugees from Nazi persecution – knew that a German group led by Nobel prize winner Werner Heisenberg was pursuing the same goal.

Two pathways seemed promising, and the Manhattan Project explored them in parallel. One group struggled to separate usable quantities of uranium-235 from the less active (but vastly more abundant) uranium-238. Meanwhile, another team led by Seaborg tried to extract plutonium-239 from reactor residues. This was difficult, and dangerous. Seaborg wrote: ’My section’s mission seemed impossible: design an automated process to mass produce an element that existed in such small quantities that no one had ever even seen it. The production would be shielded behind thick walls of concrete, and once it began no one could make repairs or adjustments without receiving a lethal dose of radiation. We had our share of setbacks. One night a shelf collapsed because a worker overloaded it with radiation-shielding lead. A vial crashed on the bench and a quarter of the world’s supply of plutonium soaked into the Sunday Tribune. Yet somehow we stayed on the ambitious schedule.’

By late 1944 it seemed that fighting would cease in Europe before either side produced a nuclear weapon, but the project continued. Its goal was now to enforce a rapid victory in the Pacific by hitting Japan with nuclear bombs. Many scientists feared this would entail massive civilian casualties. Seaborg was one of several who signed the Franck Report, which argued that demonstrating the weapon on an uninhabited island would suffice to compel a Japanese surrender. Senior officials declared this impracticable, and in August 1945 Hiroshima was devastated by a uranium bomb, Nagasaki by a plutonium one. The war ended and the nuclear age began.
Periodic puzzles
In 1946, Seaborg returned to academic life at Berkeley. While working on plutonium he had detected further transuranium elements and wanted to complete – and publish – his investigations. But alongside all the practical problems of handling these elements he faced a theoretical puzzle – where did they fit in the periodic table? His problem had a precedent. The rare earth metals, or lanthanides, had also been difficult to place within the periodic system until Niels Bohr developed his electron shell model of the atom.
In 1921, Bohr had proposed that the 14 elements following lanthanum in the table formed an inner transition series, the number of their valence electrons remaining constant at three while successive vacancies in the interior 4f electron shell were filled. He predicted that an undiscovered element immediately following this series would fit into the same group of the table as titanium and zirconium. Dirk Coster and George de Hevesy found this element, showed its properties conformed to Bohr’s expectations, and named it hafnium. (Hafnia is the Latin name for Copenhagen.) So far, so good!
Bohr also suggested that elements beyond actinium formed another inner transition series, in which the 5f electron shell was being progressively filled. Chemists were far more sceptical about this. They continued to locate thorium (atomic number 90, maximum valency 4) in group 4, along with titanium and zirconium – and now, hafnium. Meanwhile, uranium (atomic number 92, maximum valency 6) still seemed quite at home with chromium, molybdenum and tungsten in group 6.
When the first transuranium elements were discovered, it therefore seemed logical to place neptunium in group 7 with manganese and rhenium – plutonium went in group 8 with ruthenium and osmium. Seaborg’s investigations suggested otherwise, and in 1945 he published a restructured periodic table with an actinide series located directly under the lanthanides. Initially dismissed as a crazy idea by many chemists, it soon became the standard version.
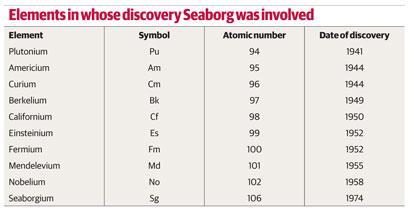
Studies of americium (atomic number 95) and curium (atomic number 96) confirmed that three was their principal valency. Further research revealed that each successive transuranium element exhibited a more stable (iii) oxidation state than its predecessor. Consequently, Seaborg argued that three was the fundamental valency of all the actinides, even though it only became the dominant one from plutonium onwards. He was also able to demonstrate similarities between each actinide and its corresponding lanthanide, which became increasingly apparent as the series progressed.
This argument rested primarily upon chemical evidence – obtained with great difficulty, since only tiny samples of the new elements were available. However, Seaborg also had a physical explanation for the higher valency states exhibited by the earlier actinides. As he noted in his 1951 Nobel lecture: ’The 5f and 4d shells lie so close together that the energy necessary for the shift from one shell to the other is in some cases within the range of chemical binding energies.’
And another one
In subsequent years, Seaborg and his co-workers used ever improving techniques to produce and investigate more transuranium elements – berkelium, californium, einsteinium, fermium, mendelevium, nobelium and finally seaborgium. But while continuing to coordinate this massive research programme, Seaborg was also heavily engaged with many other activities.

He served as Berkeley’s chancellor from 1958 to 1961 (and always remained an enthusiastic supporter of its football team). He chaired the US Atomic Energy Commission from 1961 to 1971, where he played an important part in international negotiations leading to the UN’s non-proliferation of nuclear weapons treaty. Successive presidents consulted him on important issues – Lyndon Johnson once summoned him from the swimming baths, naked and dripping, to answer an urgent question by telephone. For many years a keen golfer, Seaborg was also a dedicated wilderness hiker and environmentalist.
Seaborg remained active until the last months of his life. He suffered a stroke in August 1998 which led to his death on 25 February 1999. His name lives on among the elements, but also among the stars. In 1983, the asteroid 4856 Seaborg was named in his honour – an appropriate memorial to one who, like Star Trek’s Captains Kirk and Picard, boldly went where none had gone before.
Mike Sutton is a visiting fellow in the department of humanities of Northumbria University, UK
Further Reading
-
Handbook on the physics and chemistry of rare earths, 41, Elsevier, 2011.
-
G T Seaborg and E Seaborg, Adventures in the atomic age: from Watts to Washington, Farrar, Straus and Giroux, 2001.
-
J W van Spronsen, The periodic system of chemical elements: a history of the first hundred years, Elsevier, 1969.












No comments yet