Chris Smith
Hello – beauty, blue glass, B12 and the best magnets that money can buy. So why is this week's element named after a goblin?
Sarah Staniland
I always find the question 'what's your favourite element' a difficult one. There are several front runners for vastly varying reasons; however, always a top contender has to be cobalt because it excels in several important character traits: Cobalt has amazing beauty and strength, as well as great cooperation. All together a highly useful metal.
Before I even thought about the chemistry of colour I developed a love for blue glass, something I still collect to this day. Only after studying the transition metal chemistry did I realise that this beautiful blue colour comes from cobalt. Cobalt chloride in fact.

However, as far as colours go, cobalt has a few more strings to its bow than just this wonderful blue. Cobalt can also colour glass green, while the hydrated form of Cobalt chloride is a beautiful deep rose colour. As you can imagine this colour change due to the presence of water is highly useful, warranting cobalt chloride an ideal moisture indicator.
The array of beautiful colours that cobalt produces were never more prevalent to me than when I went to the cobalt mining region called the Copperbelt in Zambia. In this area the huge multicoloured cobalt minerals deposits tower high, with the shores of dams and streams coloured deep rose with silvery blue veins running through.

Cobalt it is not found pure in nature but found in sulphur minerals and usually associated with other transition metals. As you can probably guess from the name of the region in Zambia – the Copperbelt, cobalt is mined as a secondary product to copper that is dominant in the ore of this region. Because of this cobalt is normally recovered from the waste of the primary metal extraction.
However these mining hotspots are not the only places on the Earth where high concentrations of cobalt can be found. A huge reserve of several transition metals (including cobalt) can be found in strange nodules on the floors of the deepest oceans. The nodules are manganese minerals that take millions of years to form, and there are many tonnes of cobalt present in this form.

So you can see that cobalt is never found alone but always palled up with other transition metals in their ores, mainly copper and nickel. In fact cobalt metal was not isolated and purified until as late as 1735 by the Swedish scientist G. Brandt.

Cobalt can also sometimes be found in mixed arsenic ores, and it is cobalt's association with arsenic that gives it its name. The word cobalt comes from the German 'Kobolds' which means goblin or trouble maker. It was so called in this early mining region because it was very difficult to smelt without oxidising and smelting would release the associated arsenic vapours which would lead to pretty troublesome or even deadly processing conditions for the worker. The Kobolds were blamed and the name stuck.
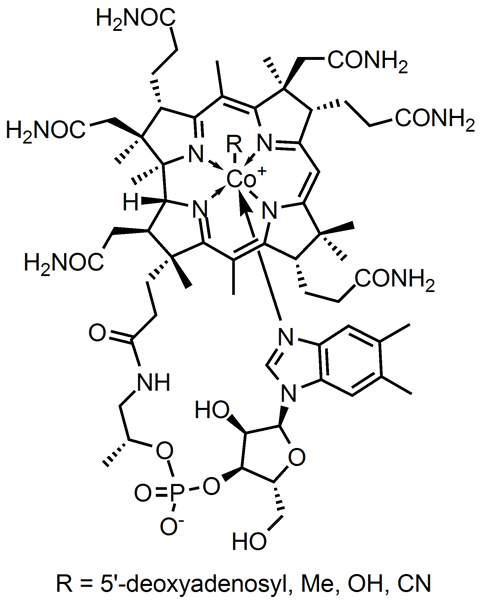
With the exception of the mining region, cobalt is not very abundant, with only trace amounts in the Earth's crust (about 2500 times less than iron). However, it is a metal that is essential for life in the trace amounts. Cobalt is the metal at the centre of vitamin B12 which helps regulate cell development and therefore DNA and energy production in the body.
Cobalt has been known and used by people for its beautiful colouring and pigment properties as far back as 2500BC. Egyptian cobalt blue paints and Prussian cobalt oxide necklaces have been dated back to this time while cobalt glass has been found in a Greek vase dated at 100 BC. Cobalt was also used to colour glass in the Chinese Tang dynasty from 618 AD. In fact all the way up until the beginning of the 20th century people have only really exploited cobalt for its beautiful colour.

However cobalt is not just a pretty face. Cobalt is a lustrous very hard silvery metal belonging to a group called the transition metals. It is one of only 3 ferromagnetic transition elements along with iron and nickel. As a metal it is very mechanically hard and tough, and it has a very high melting point (hence the smelting problems) and also remains magnetic to the highest temperature of all the magnetic elements.
When cobalt is combined with other metals its strength allow a range of super alloys to be created. In particular, cobalt's very high melting point and mechanical strength at high temperatures has seen its extensive use in what is termed 'superalloys'. These are alloys that retain mechanical strength at high temperatures. Because of its impressive properties cobalt is an important component in wear resistant and corrosive resistant alloys. And cobalt alloys and coatings are seen everywhere from drills to saws, from aircraft turbines to prosthetic bone replacements.

The fact that cobalt is magnetic has also been exploited with the Japanese invention of cobalt magnetic steel where adding cobalt to steel vastly increases the magnetic hardness. Just a few years after that in the 1930s saw the pivotal invention of Alnico magnets, which as the name suggests, are composed of aluminium, nickel and cobalt.
The fact that cobalt retains its magnetism up to high temperatures is also a very favourable trait when the addition of cobalt to a magnetic material can improve its properties at high temperatures. More recently the creation of rare-earth magnets have given us much stronger, harder, permanent magnets than Alnico magnets. One such magnetic material, samarium cobalt retains its magnetism up to 800°C. Because it is magnetically and mechanically hard up to very high temperatures, it has found uses in high-speed motors and turbo machinery. More recently cobalt has a major use in newer batteries, magnetic particles for recording and storage information in magnetic tapes and hard drives.

So cobalt; giving joy in an array of beautiful colours, but also ultra strong, hard and magnetic. Cobalt is never alone, it is found associated with different metals in their ore and has its best mechanical properties when palled up with others.
Chris Smith
Emphasising the importance, of course, of teamwork. That was Sarah Staniland with the story of Cobalt – she's based at the University of Leeds. Next week it's the turn of the stuff that amongst other things makes Parker pen nibs write so nicely, but if you haven't heard of it before, then you're probably in good company.
Jonathan Steed
Stop the proverbial 'man in the street' and ask him what ruthenium is and the chances are he won't be able to tell you. Compared to the 'sexier elements' that are household names like carbon and oxygen, ruthenium is, frankly, a bit obscure. In fact even if your man in the street was wearing a lab coat and walking on a street very close to a university chemistry department he might still be a bit ignorant about this mysterious metal. It wasn't always that way, though.
Chris Smith
And you can hear how ruthenium rose to prominence with Jonathan Steed on next week's Chemistry in its element. I'm Chris Smith, thank you for listening and goodbye.












No comments yet