Support network aids performance by isolating catalytic metal
A catalytic system consisting of individual cobalt atoms anchored onto a framework can oxidise primary alcohols to aldehydes with high activity and excellent selectivity, new research shows. The system could work as a precious metal substitute when synthesising almond flavour compound, benzaldehyde.
Benzaldehyde is important for the synthesis of fine chemicals, with its applications ranging from pharmaceuticals and food flavourings to pesticides. A common method of producing benzaldehyde is to oxidise benzyl alcohols. Typical catalysts for this process contain precious metals, such as ruthenium and palladium, and while they perform very well, the cost and scarcity of these elements is problematic.
Now, a team at Tsinghua University in Beijing, China, led by Dingsheng Wang has reported a single atom catalyst based on cheap materials that can perform this reaction with outstanding results.
As the name would suggest, single atom catalysts are a class of catalyst where the active site is a single atom. They usually take the form of individual atoms dispersed on a support material that confers stability. This arrangement leaves the atoms more exposed to reactants than they would otherwise be, making for more efficient catalysis. Microscopic and spectroscopic techniques can ensure the atoms remain separate and do not aggregate into nanoparticles.
The precursor to the catalyst used by Wang’s team is a bimetallic metal–organic framework (BMOF) coated in an organic polymer. Where traditional MOFs consist of metal atoms of a single type connected by an organic linker, BMOFs contain two different metals – in this case cobalt and zinc. ‘BMOFs can not only be used as the precursor of the single metal atom species, but can be employed as the precursor of the support,’ explains Wang. Pyrolysis of the BMOF–polymer composite results in the final catalyst.
For the oxidation of benzyl alcohols to benzaldehyde, this catalyst demonstrates near perfect performance, with conversion and selectivity figures of 99%. This means that the reaction uses up almost all of the starting material while almost completely avoiding unwanted side-products. Sir John Meurig-Thomas, a catalysis expert based at the University of Cambridge in the UK, is impressed by these results: ‘This is a very commendable piece of work in producing highly active, highly selective single atom catalysts.’
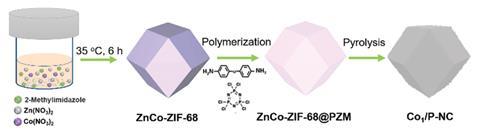
Despite the exceptional performance, the complexity of the method used to produce the catalyst could be a barrier to its large-scale application. According to Meurig-Thomas, ‘I do think if you’re trying to commercialise something like this, you would need to have a rather more streamlined and simpler method of producing a solid support that keeps the individual cobalt atoms separate.’
References
S Ji et al, Nanoscale Horiz., 2019, DOI: 10.1039/c9nh00036d










1 Reader's comment