Chemical bonding – Page 5
-
 Research
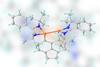
ResearchSynthetic molecules fold up into abiotic proteins
Compound that self-assembles into giant folded ring could help scientists design bespoke abiotic proteins
-
 Research
ResearchLongest silicon—silicon double bond has two-faced reactivity
First hypercoordinated disilene’s extra-long silicon–silicon double bond gives it ambivalent reactivity
-
 Research
ResearchNewest tetrel bond is five times stronger than its peers
First intermolecular three-centre four-electron bond could unravel fleeting intermediate’s nature
-
 Research
ResearchBonding rethink called for as new metavalent bond proposed
Combination of elements in the metalloid region of periodic table produces a bond with both metallic and covalent characteristics
-
 Research
ResearchWorld record for longest carbon–carbon bond broken
Carborane bond surpasses previous longest bond after just nine months in the top spot
-
 Research
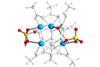
ResearchMolecular decoration determines origin of MOF acidity
Analytical combo used to pinpoint strong Brønsted acid site in promising next-generation solid acid catalyst
-
 Research
ResearchHydrogen sulfide surprises as it's discovered to have hydrogen bonds
Nobel laureate Linus Pauling was wrong – H2S does form hydrogen bonds after all
-
 Research
ResearchRules to distinguish between tetrel and hydrogen bonds
Quantum calculations reveal subtle but significant geometric differences
-
 Research
ResearchInorganic molecule mimics odd benzene isomer
Zwitterionic boron–nitrogen compound has σ bond between two π orbitals
-
 Opinion
OpinionThe bonds that bind
Chemical bonds continue to fascinate chemists – and bring us together too
-
-
 Research
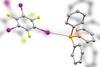
ResearchMacrocycles power up carbon nanotubes
Interlocked molecules tune the electronic properties of nanotubes, allowing researchers to control their catalytic activity
-
 Research
ResearchBeryllium double bond predicted
Proposed Be–Be bond would be first double bond between s block elements using only π electrons
-
 Research
ResearchFirst arsenic–germanium double bond made
Scientists use push–pull substitution to synthesise heavy imine
-
 Research
ResearchValence bond theory probes fundamental nature of hydrogen bonding
New insight reignites covalent versus electrostatics debate
-
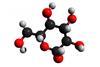 Research
ResearchPuzzle of why very similar sugars can taste much sweeter than others solved
The sweetest saccharides form the strongest and shortest hydrogen bonds
-
 Research
ResearchNanotube locked inside a porphyrin
Rotaxane-like assembly formed from mechanically interlocked carbon nanotubes and macrocylic porphyrin rings
-
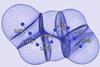 Research
ResearchDelocalisation pins down meaning of bond order
Reliable bond order definition provides new insights into covalency
-
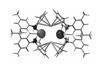 Research
ResearchAnionic aluminium turns textbook knowledge on its head
First stable nucleophilic aluminium(I) compound offers new way to make aluminium–carbon bonds
-
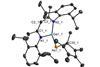 Research
ResearchIntroducing gallaarsene, the first of its kind
Molecule features rare gallium–arsenic double bond