Drugs that change how your genes are switched on or off could change how we treat many diseases, as Rachel Brazil discovers
Drug discovery is catching up with epigenetics. The chemical modifications of the genome that do not involve changes to the underlying DNA sequence provide a genetic control system which is now understood to be at the root of many cancers, neurodegenerative and immune disorders. Chemists are now in hot pursuit of epigenetic targets and drug molecules. A new generation of these drugs is on the way and it looks like they will be able to treat a wide spectrum of diseases using a variety of inventive strategies.
Epigenetics describes the chemical system by which biology controls how the genetic blueprint is used in different types of cells – which genes are switched on and off, and therefore which proteins are expressed to make, say, a liver cell or a heart cell. The best understood epigenetic modification is DNA methylation, which occurs predominantly on the C5 position of cytosine bases.
Methylated cytosine is found all over the genome, but there are ‘gene promoter’ regions where methyl groups are missing, and where transcription – the process by which the DNA code is read to make proteins – starts. Transcription factors bind to the DNA and a gene is switched on, but if the cytosine is methylated, they are prevented from working and the gene is switched off.
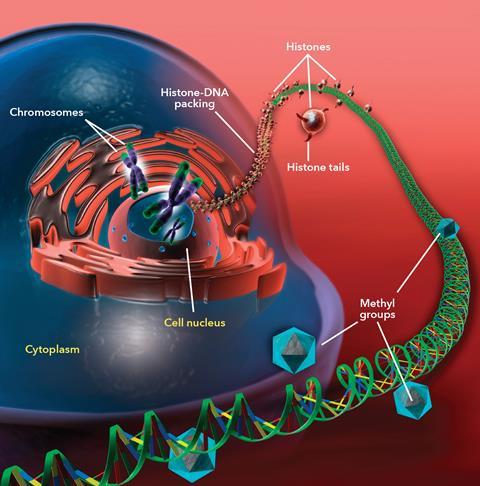
The other well-known epigenetic process involves histones – the proteins around which DNA is wrapped to form chromatin, a structure often described as looking like ‘beads on a string’. If a histone is chemically modified, by adding or removing acetyl or methyl groups to a lysine amino acid, the chromatin structure changes, affecting access to genes.
Genetic switches
Epigenetics controls the way biology interacts with its environment. Think about the honeybee: all bees in a hive share the same genome, but fed different food, they develop differently. Those fed on nectar and pollen turn into male drones or sterile female workers, but one lucky larva who gets continuously fed royal jelly turns into the queen. Royal jelly contains compounds that are capable of blocking the methylation of cytosine bases in honeybee DNA, and these changes result in a queen bee five times larger than the workers.

It’s not a coincidence that the conditions so far tackled by epigenetic drugs are often attributed to environmental factors like bad diets or smoking. Scientists now think diseases like cancer, diabetes and Alzheimer’s are not only dependent on genetic factors, but on epigenetic changes caused by environmental factors. This is leading to new druggable targets. ‘Epigenetic events driven by a whole suite of different enzymes and regulatory processes in theory could be inhibited or even maybe reversed by using suitable small-molecule inhibitors,’ says Matthew Fuchter, a medicinal chemist at Imperial College London, UK.
Fuchter’s group is working on several epigenetic approaches, all based on modulating the enzymes responsible for maintaining the so-called epigenetic marks on DNA and histones. They come in three categories: ‘writers’, such as DNA or histone-methyltransferases which attach methyl groups to DNA or histones; ‘erasers’, including histone deacetylases that remove acetyl groups from histone proteins; or ‘readers’, like acetyl lysine recognition proteins that bind to the marks themselves, promoting gene expression.
From the mid-2000s the first epigenetic cancer drugs appeared, and there are currently five histone deacetylase (HDAC) inhibitors and two DNA methyltransferase (DNAMT) inhibitors approved. ‘Because epigenetic targets are a relatively new class, there has proved to be quite a lot of challenges in identifying robust start points,’ Fuchter says. The other problem is that the results of modulating epigenetic pathways is not always predictable, due to biology’s habit of building in redundancies. ‘You could view individual epigenetic marks as contributing to layers of packaging in the regulation of gene expression. It is not always immediately apparent what is the most important epigenetic target involved,’ explains Fuchter. ‘If you inhibit one epigenetic target you don’t necessarily see the breadth of effects one might expect.’ Nevertheless, a growing pipeline of candidates is now developing from biotechs and big pharma: a 2015 estimate suggested around 90 companies are developing over 100 epigenetic therapies in cancer, for over 50 separate targets.
Paul Brennen, a medicinal chemist at the University of Oxford, UK, is using chemical probes to find epigenetic drugs. He thinks they have helped the field massively. ‘Because what we see with the inhibitors is not always what you would predict ahead of time looking at the biology.’ Small-molecule probes may not have full drug-like properties, but their interactions with target proteins can help to understand the relationship between epigenetic marks and the underlining genes that are being switched on or off. This is done using a technique known as ChIP-Seq: chromatin immunoprecipitation (ChIP) uses antibodies attached to magnetic beads to precipitate out a chromatin protein of interest and the accompanying DNA is then sequenced to identify the gene.
The HDAC inhibitors, which have so far been the major focus of epigenetic drug discovery, increase chromatin acetylation levels, generating an open chromatin state that leads to the expression of previously silenced genes. This in turn results in a more normal cell cycle and cancer cell death. The first generation of HDAC inhibitors lacked selectivity and tended to target the whole family of around 18 HDAC enzymes. Their broad action causes side-effects such as vomiting, immune suppression and lack of appetite.
‘Wake and kill’
Now a second generation of drugs are in the pipeline that are more selective in design. Epizyme, a start-up based in Cambridge, US, is also taking advantage of how cancer cells are often dependent on epigenetic pathways for their survival.The eight-year-old company’s lead drug tazemetostat, now undergoing clinical trials to treat multiple types of cancers, is a small molecule inhibitor of the histone methyltransferase EZH2.
What seems to happen in some cancers is that mutated histone methylation enzymes target the wrong genes, which results in abnormal cancer cell proliferation. ‘Our approach is to look for a situation where a pathway is disregulated in such a way that the cancer cell is addicted to the enzymatic activity of a specific histone methyltransferase in a way that is not shared by normal cells, so that inhibiting that histone methyltransferase would be fatal to the cancer cell, but would would be benign to a normal cell,’ Epizyme’s chief scientific officer, Robert Copeland explains. The inhibitor is able to block the specific enzyme, but as the histone methyltransferase plays a negligible role in normal cellular activity, it is possible to have a selective impact, with minimal effect on non-cancer cells.
We are able to kill those cancer cells
Robert Copeland
Copeland says the mutated EZH2 enzyme is found in up to 30% of patients with non-Hodgkin lymphoma but that other patients could also benefit from the type of inhibitors Epizyme is designing. Some cancers are caused by mutations in proteins other than a histone methyltransferase, but still lead to genes being switched on or off; in this state, the cell nevertheless becomes reliant on a histone methyltransferase enzyme pathway to proliferate. ‘We’ve seen if we then come in with an EZH2 inhibitor – even though the mutation is not within the EZH2 enzyme – we are able to kill those cancer cells,’ adds Copeland.
Cancer still dominates the epigenetic drug discovery landscape, but attention is turning to broader groups of diseases. Fuchter is looking at how epigenetic pathways could be used in the fight parasitic diseases like malaria – by focusing on the plasmodium parasite’s own epigenome. ‘This parasite, just like humans, relies on epigenetic events to regulate transcription, and therefore you can design compounds to interfere with the epigenetic processes in the parasite,’ he says.
One significant problem in treating malaria is the ability of certain species of plasmodium to stay dormant in the liver, undetected and drug-resistant. At some point, months or years later, it will activate to cause further bouts of malaria. But as Fuchter explains, ‘Epigenetic processes in plasmodia are hypothesized to control transcription across various different life cycle stages of the parasite and so these targets potentially provide opportunities that wouldn’t necessarily be available using conventional anti-parasitic compounds.’
In 2014, Fuchter and a group of international collaborators discovered plasmodium histone methyltransferase inhibitors that could not only kill the blood stage parasite, but also ‘reawaken’ the dormant liver stage. The reawakened parasites could then be tackled by the usual anti-malarial drugs – in what Fuchter calls a ‘wake and kill’ parasite combination therapy.
Turning off addiction
Another possibility offered by epigenetic drugs is the ability to treat some conditions with one-off medicines that effectively reset the epigenome. Moshe Szyf, a pharmacologist at McGill University in Montreal, Canada, has been testing such an approach for treating cocaine addiction. One of the pioneers of epigenetics, Szyf has been working in the field for over 30 years and has now turned his attention on how to reverse changes that occur in the epigenomes of addicts during withdrawal. These give rise to increased cravings and often cause relapse into drug-use. For years, researchers have been looking for the genes responsible for addiction, but now it seems like epigenetics may play a big role.
Szyf and his collaborator Gal Yadid from Israel’s Bar Ilan Univeristy started by working out how cocaine addiction changes DNA methylation levels in rats. They found that during withdrawal – not during exposure to the drug itself – the biggest changes to DNA methylation occur, with hundreds of genes becoming methylated. To try and stop the addictive behavior, Szyf experimented with an epigenetic drug – a small molecule DNA methylation inhibitor: RG108.

Cravings were tested after 30 days of withdrawal with rats trained to self-administer cocaine using light or sound cues. Addicted rats developed intense drug-seeking behaviour when exposed to the cue, but if the drug RG108 was injected just before animals were exposed to the cue, the addiction stopped permanently – suggesting a single treatment reprogrammes the epigenetic marks and effectively ‘cures’ the drug addiction. ‘It stunned us that, different to many other pharmacological treatment that have been used to treat addicted animals, you do one treatment and it’s gone,’ Szyf says.
‘We have learned over the years that addiction is not caused by one gene, it is caused by networks that change their outputs from one state to another, so the challenge in medicine is not to develop a specific antagonist – in most cases it’s futile because the network has so many other ways to by-pass that – but to reprogramme the network. I think that’s where these kinds of drugs will be crucial,’ explains Szyf.
Another curious effect has been documented with epigenetic drugs – in this case, to treat Huntington’s disease – a fatal inherited, degenerative condition that causes a loss of motor skills and cognitive impairment. Beth Thomas, a neuroscientist at The Scripps Research Institute in California, US, has been testing a series of more selective HDAC inhibitors synthesised by her Scripps colleague Joel Gottesfeld. ‘She has found that not only can these drugs lessen the symptoms and delay the onset of Huntington’s disease in mice, but that they can provide improvements to the male offspring of those mice treated with the drug, who also carry the Huntington’s disease gene.’
As well as working as a histone deacetylation inhibitor, experiments on mouse brain and muscle samples confirmed methylation changes were also caused by the drugs, particularly on the Y-chromosome, which may explain why the biggest improvements were seen in male offspring. ‘It seemed to be several different epigenetic mechanisms going on,’ says Thomas. ‘People know that DNA methylation is a stable mark that can be inherited in the genome, so then we asked a question, I wonder if this effect can be inherited.’
An inherited problem
The idea of inheriting epigenetic marks was once controversial, as it was thought that the genome was largely wiped clean prior to embryonic development. It’s now clear that this isn’t the case, with growing evidence that epigenetic changes caused by environmental stresses such as famines are carried forward. A study carried out in 1996 analysed the children of the Dutch population who experienced the 1944 ‘hunger winter’ when a German blockade cut off their food supplies for six months. Researchers found that these people had a whole host of increased health risks such as cardiovascular and metabolic disease. But ‘not every event has to be detrimental’ says Thomas. ‘Our paper was interesting as we found the [transgenerational] effect beneficial.’
Given that epigenetic therapies are making changes to something as fundamental as how our genetic code is read and can sometimes be passed on to the next generation, should we be more worried about the long-term safety of epigenetic drugs? ‘Our phase one experience has been quite good in terms of patient safety and tolerability,’ says Copeland, and Brennen agrees that current safety testing is sufficient to pick up problems.
But the idea that a drug might have permanent effects that can be passed onto your children does seem alarming – although in some passing down protection may be beneficial. But this is the case for more than just epigenetic drugs: a lot of what people do in their everyday lives, including smoking and their diet, has the potential to alter their epigenome.
‘Epigenetic drugs should never ever be used on pregnant mothers but I think for other adults there are very little side effects,’ argues Szyf. But he admits their relative safety does seem counterintuitive. ‘You would think if you treat a patient with a drug which changes DNA methylation all over the place, you will completely change that person – but it doesn’t happen,’ he says. He suggests this is because it’s hard to change a cell’s underlying ‘authentic programme’ because of all the mechanistic layers that back it up. ‘I think the robustness of our methylation programmes are very hard to change, whereas the experiential add-ons are much easier to change and that creates a selectivity,’ Szyf says.
So what kind of future pharmaceuticals can we expect from epigenetic drugs? For example, will we be able to use medicines to reverse the damage to our epigenomes caused by our poor lifestyle choices? We know that smoking tobacco causes changes in the methylation levels to smokers’ DNA and the likelihood is that these changes play a role in the link between smoking and cancer. So could a drug reprogramme smokers’ epigenomes to remove the risk of disease? Could we keep on eating hamburgers and chips and take a pill to reverse the epigenetic damage?
’It’s certainly a theoretical possibility,’ says Copeland. ‘We would need a much better understanding of the interplay of all the different epigenetic modifications and all the regulatory networks that link them to have a fighting chance of doing something like that,’ says Fuchter. ‘My guess is that it won’t be possible for an awfully long time.’
Rachel Brazil is a science writer based in London, UK












No comments yet